
TNM stages inversely correlate with the age at diagnosis in ALK-positive lung cancer
Introduction
Non-small cell lung cancer (NSCLC) is increasingly understood to be a heterogeneous disease (1,2). Rearrangements of the anaplastic lymphoma kinase (ALK) gene are present in 3–7% of NSCLC patients (3). ALK rearrangements define a distinct subgroup of NSCLC that typically occurs in young patients who have never smoked and who have adenocarcinoma histological characteristics (4-6). Several studies have demonstrated that the ALK gene has a high incidence in advanced NSCLC (7,8). However, there was no consensus on the frequency of surgical patients with ALK-positive lung cancer (9-11). Among NSCLC patients with a targetable genomic alteration, it suggested that younger patients were associated with an increased likelihood of initially presenting with stage IV disease (11,12) and exhibiting a poorer survival than older patients (12).
To clearly investigate the associations between age, tumor-nodes-metastasis (TNM) staging and frequency of ALK-positive lung cancer, we performed an analysis of ALK-positive lung cancer cases in a large-scale cohort and compared to the results to those of another clinically relevant cohort: Kirsten rat sarcoma viral oncogene homolog (KRAS)-mutant lung cancer cases.
Methods
Patients
The clinical records of 8,405 consecutive patients with lung cancer who had ALK detection and 2,187 consecutive patients with lung cancer who underwent KRAS testing at Guangdong Provincial People’s Hospital (GDPH) between September 2010 and January 2018 were retrospectively reviewed. This study was approved by the Ethics and Scientific Committees of Guangdong Provincial People’s Hospital [No. GDREC2016175H(R2)]. In our center, patients with lung cancer were routinely tested for ALK gene rearrangements. Of the 8,405 ALK screening patients, 3,782 patients had stage I–IIIa and 4,623 had stage IIIb–IV disease. Among the patients who underwent KRAS testing, 834 patients and 1,353 patients had stage I–IIIa and stage IIIb–IV disease, respectively (Figure 1). ALK was assayed by immunohistochemistry (IHC), fluorescence in situ hybridization (FISH) or next-generation gene sequencing (NGS). The age at initial diagnosis was extracted. In the resected patients, the T and N staging were from the results of surgical resection, and TNM staging in unresectable patients was based on the comprehensive imaging results. The T, N, and M stages were classified according to the International Association for the Study of Lung Cancer (IASLC) 7th TNM staging project. The mean age was compared between the ALK-positive and KRAS-mutant patients at various TNM stages. The patients were divided into the following four groups stratified by age: <40, 40–49, 50–59 and ≥60 years; the clinical features and survival of the different age groups were analyzed. Stage I–IIIa was usually defined as resectable or potentially resectable and stage IIIb–IV was considered to have no curative treatment. For each included patient, we collected the following data: age; sex; smoking history; pathology; Eastern Cooperative Oncology Group (ECOG) score; TNM stage; presence of brain metastasis at initial diagnosis; major treatments including surgery; targeted therapy; chemotherapy or/and radiotherapy; and overall survival (OS).

Statistical analysis
Statistical analyses were performed using SPSS (version 20.0; SPSS Inc., Chicago, IL, USA). The Chi-square test or Fisher’s exact test was used to compare categorical variables, and the Wilcoxon rank sum test was used for continuous variables. The Spearman correlation test was applied to assess the relations between age and clinical stages or various TNM categories. Furthermore, a linear regression test was used to estimate the trend between the percentage of stage IIIb–IV disease in various age groups. Univariate and multivariable Cox proportional hazards models were used to identify prognostic factors for survival. OS was defined as the time from the initial diagnosis until death from any cause. Survival curves were constructed using the Kaplan-Meier approach and compared using the log-rank test. A two-sided P value of <0.05 was considered statistically significant.
Results
Patient characteristics
In the ALK-positive cohort, 411 (4.9%) eligible patients were identified, including 127 patients with stage I–IIIa disease and 284 patients with stage IIIb–IV disease (Figure 1). Overall, 383 (93.2%) patients had histologically confirmed adenocarcinoma. Of the 411 eligible patients, there was almost an equal proportion of females [n=204 (49.6%)] and males [n=207 (50.4%)] in our study, and 321 (78.1%) patients in our cohort had never smoked. The ECOG scores of the patients were primarily low (score =0–1) [n=380 (92.5%)]. Moreover, the majority of ALK-positive patients had an absence of brain metastasis at the initial diagnosis [n=349 (84.9%)]. Of the treatment strategies, 129 (31.4%) patients underwent surgery, 180 (43.8%) patients received targeted therapy, and 150 (36.5%) patients were treated with chemotherapy or/and radiotherapy (Table 1).
Full table
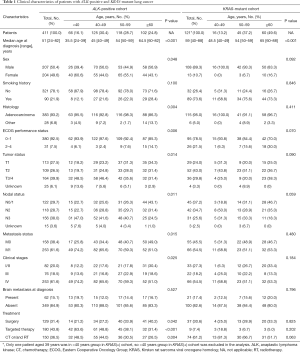
The median age at diagnosis of the patients included in ALK-positive cohort was 51 years (range, 24–82 years). As shown in Table 1, young patients with ALK-positive lung cancer were associated with a high likelihood of being female (P=0.048), having histological adenocarcinoma characteristics (P=0.004), and exhibiting low ECOG scores (P=0.006). In addition, young patients more frequently had diseases in the T3/4 stage (P=0.014), lymph node metastases (P=0.011) and distant metastasis (P=0.015) (Table 1) than old patients.
There were 122 (5.6%) patients in the KRAS-mutant cohort who had a median age at diagnosis of 59 years (range, 40–88 years), including 46 patients with stage I–IIIa disease and 76 patients with stage IIIb–IV disease (Figure 1). As shown in Table 1, there were no significant differences in the clinical characteristics between the various age groups.
Association between age at diagnosis, frequency and TNM stages
Among all patients with ALK-positive lung cancer, the mean age at diagnosis decreased steadily with more advanced clinical stages [I/II vs. III vs. IV, mean age ± standard deviation (SD): 54.7±11.4 vs. 52.0±10.5 vs. 49.7±11.6 years]. There was a significant difference in age at diagnosis between the various clinical stages (P=0.002), and the age at diagnosis inversely correlated with the clinical stages (P<0.001) (Figure 2A). Moreover, these associations also existed for T stages (T1 vs. T2 vs. T3/4, mean age ± SD: 53.2±10.7 vs. 52.7±11.0 vs. 49.4±12.1 years), N stages (N0/1 vs. N2 vs. N3, mean age ± SD: 53.6±11.8 vs. 51.7±11.0 vs. 49.2±11.4 years), and M stages (M0 vs. M1, mean age ± SD: 53.4±11.0 vs. 49.7±11.6 years). Significant differences were also observed in age between various T, N, and M stages (T stages: P=0.003; N stages: P=0.004; M stages: P=0.001) and the age at diagnosis inversely correlated with the T, N, and M categories (T stages: P=0.001; N stages: P=0.001; M stages: P=0.001) (Figure 2B,C,D). However, the KRAS-mutant patients did not demonstrate similar characteristics to those of ALK-positive patients. The mean age at diagnosis of KRAS-mutant patients was 63.0±8.9, 58.1±9.6, 59.4±10.5 years for stage I/II, III, IV disease, respectively. There was no significant difference in age at diagnosis between various clinical stages (P=0.064) and there was no significant inverse correlation between age at diagnosis and clinical stages (P=0.084) (Figure 2A). Although the age at diagnosis showed a significant inverse correlation with the N categories (P=0.010), the correlation was not significant in the T (P=0.887) or M categories (P=0.152) (Figure 2B,C,D).
Furthermore, the proportion of ALK-positive lung cancer that was stage IIIb–IV disease decreased steadily as the age groups became older (84.8% vs. 73.6% vs. 66.1% vs. 56.9%) (F=338.4; P=0.003). However, the KRAS cohort did not show this significant linear relation (F=10.6; P=0.190) (Figure 2E). In this study, the total frequency of ALK rearrangements was approximately 4.9%. We found that the frequency of ALK rearrangements in patients with stage IIIb–IV disease was much higher than that of the patients at stage I–IIIa disease (6.1% vs. 3.4%, P<0.001). However, the frequency of the KRAS mutation in patients with stage I–IIIa and IIIB–IV disease were similar at 5.5% and 5.6%, respectively (P=0.924) (Figure 2F).
Survival analysis
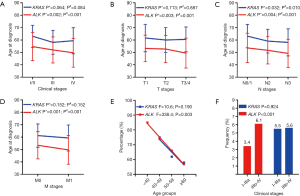
The median follow-up time was 13.7 months (range, 0.1–95.8 months), and the last follow-up was recorded on August 10, 2018. In the ALK-positive cohort, 112 (27.3%) patients died during follow-up. The 5-year OS rates were 40.5%, 47.8%, 44.8% in the <40 years, 40–49 years and 50–59 years groups, respectively. Among the ALK-positive patients aged 60 years or older, the 5-year OS rate reached 65.6%. There were statistically significant differences in OS between the ≥60 years group and <40 years group (P=0.048) and between the ≥60 years group and the 50–59 years group (P=0.041). Although the OS between ≥60 years group and the 40–49 years group was not significantly different (P=0.135), the patients in the ≥60 years group were associated with a trend toward better survival compared to the other age groups (Figure 3A and Table 2). However, in the KRAS cohort, there were no significant differences between the three age groups (40–49 vs. 50–59 years, P=0.052; 40–49 vs. ≥60 years, P=0.205; 50–59 vs. ≥60 years, P=0.451) (Figure 3B and Table 2).

Full table
For the ALK-positive cohort, multivariable analyses revealed that non-intracranial metastatic disease [hazard ratio (HR): 2.87; P<0.001] and the presence of brain metastases at diagnosis (HR: 3.72, P<0.001) were associated with poor survival, as was the presence of high ECOG scores (HR: 3.97; P<0.001). Furthermore, non-intracranial metastatic disease (HR: 4.90; P<0.001) and the presence of brain metastases at diagnosis (HR: 4.76, P<0.001) were also prognostic factors in KRAS-mutant patients (Table 3).

Full table
Discussion
The rearrangements of ALK defined a molecular subset of NSCLC with distinct clinical and pathological features. The included patients shared similar clinical features, including never/light smoking history, adenocarcinoma, and young age (4,6,13). However, few large-scale studies have clearly explored the correlations between the age, TNM stage and frequency of ALK-positive lung cancer.
In the most recent decade, several small-scale studies of this subgroup described the frequency of ALK-positive lung cancer in young patients or groups of patients stratified by age. In addition, some surgeons also explored the proportion of ALK rearrangements among the surgical population. These studies are summarized in Table 4 (4,5,8-19). As shown in Table 4, the frequency of ALK-positive lung cancer in the younger group ranged from 10.1% to 40.7% and was much higher than that of the older group, which ranged from 0.9% to 4.2%. Tanaka et al. (11) and Sacher et al. (12) demonstrated that the likelihood of exhibiting ALK translocations steadily decreased with age. In this study, we also identified this downtrend in a large-scale population. Regarding the difference in frequencies between TNM stage groups, few studies have focused on the proportion of ALK mutations in advanced lung cancer. However, the ALK rearrangements were more frequent in stage IV disease, with a high rate that ranged from 9.7–28.0% (7,8,14), than in the unselected population (3), which had a rate that ranged from 3–7%. In contrast, the frequency of ALK-positive lung cancer varied from 1.0% to 9.0% in the surgical patients with relatively early-stage disease. Yip et al. performed genotyping profiles of resected tissues from 204 patients with stage IB primary lung adenocarcinoma and found that only 2 (1%) patients exhibited ALK rearrangements (9). However, Zhou et al. reported that the frequency of EML4-ALK fusions was 9.0% in 134 stage IA NSCLC cases (10); Blackhall et al. compared the difference between detecting for ALK with IHC and Fish in 1,281 European surgical patients with stage I to III adenocarcinoma, and reported two distinct frequencies of 6.2% and 2.2%, respectively (5). The various results may be attributed to multiple confounding factors, including small-scale patient samples, differing diagnosis technology, selected patients with a young age and nonsmoker. In our hospital, testing for ALK is a routine examination for patients treated with surgery. Our results demonstrated that the frequency of ALK mutation was 3.4% (127/3,782) in stage I–IIIa patients. However, in our results, the ALK-positive patients had a 79% increased likelihood of exhibiting stage IIIb–IV disease at the initial diagnosis, whereas, the KRAS-mutant patients did not demonstrate these special characteristics.
Full table
Of note, we identified with the Spearman correlation test that the TNM stages exhibited an inverse correlation with age at diagnosis, which suggested that patients who were younger at the initial diagnosis had a greater likelihood of being diagnosed with a more advanced diseases than patients who were older. A retrospective study by Liu et al. analyzed the clinicopathological features of ALK fusion in 200 advanced NSCLC patients. The median age was 48 years in the ALK-positive group (14). Nevertheless, the ALK-positive patients with early-stage disease exhibited an older median age of 55–59 years than the patients with late-stage patients (5,13,17,18). In fact, previous studies have indicated that young patients have a high proportion of stage III–IV disease in unselected NSCLC that ranges from 74% to 97% (8,11,12,20-23), which suggests that younger NSCLC patients exhibit more progressive biology than older patients. The correlation between age and TNM stages could be explained by the steady downward trend of frequencies with age and the high incidence of stage IIIb–IV disease in ALK-positive lung cancer.
In fact, we hypothesized that ALK rearrangements are involved in different biological activities at the early and advanced stages in the course of the tumor evolution, which features a period of rapid growth from the early stage into the advanced stage. Therefore, most patients with ALK mutations were diagnosed with advanced disease at the initial diagnosis. This process could explain the low incidence of ALK rearrangements in patients with ground-glass opacity (GGO) (13), but patients with GGO tended to present with more lymph node metastases than patients with ALK-negative lung cancer (17) and have a shorter recurrence-free survival (RFS) than EGFR-mutant patients (24). Furthermore, it has been supported that EML4-ALK-positive patients were observed to have more extrathoracic metastases including brain metastases at the initial diagnosis than EGFR-mutant patients (25) and patients with ROS1 gene rearrangements (26).
The realization that NSCLC in young patients is a genetically unique disease naturally (11,27) lends itself to the question of whether the natural history and underlying disease biology of NSCLC is also distinct in this subgroup. Similarly, the difference in disease biology between younger and older patients in ALK-positive lung cancer was previously unknown. Our study found that patients aged 60 or older were associated with a trend toward improved prognosis compared with the other younger groups. Therefore, age may be an independent factor to predict the frequency of ALK-positive lung cancer, disease biology and prognosis of patients with ALK-positive lung cancer.
In interpreting these findings, some inherent limitations in this study must be considered. The patients included our study were drawn from a single institution and the duration of follow-up was short. Additionally, the screening methods for detecting the ALK gene varied, and included IHC, Fish, and NGS, which led to false positives.
Conclusions
Despite the aforementioned limitations, the findings of this study expanded the understanding of the associations between age, frequency of ALK-positive lung cancer and TNM stages in ALK-positive lung cancer. Younger ALK-positive patients exhibited a higher frequency but tended to have more advanced disease than older patients. The combination of opportunity and risk requires the unique and precise therapeutic strategies.
Acknowledgements
Funding: This work was supported by Key Lab System Project of Guangdong Science and Technology Department-Guangdong Provincial Key Lab of Translational Medicine in Lung Cancer (grant No. 2012A061400006/2017B030314120, to YL Wu); Health Collaborative Innovation Major Project from Guangzhou Science and Technology Bureau (grant No. 201400000001-2, to YL Wu); Project of National Natural Science Foundation (grant No. 81673031, to WZ Zhong).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics and Scientific Committees of Guangdong Provincial People’s Hospital [No. GDREC2016175H(R2)].
References
- Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535-46. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. New Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247. [Crossref] [PubMed]
- Blackhall FH, Peters S, Bubendorf L, et al. Prevalence and Clinical Outcomes for Patients With ALK-Positive Resected Stage I to III Adenocarcinoma: Results From the European Thoracic Oncology Platform Lungscape Project. J Clin Oncol 2014;32:2780-7. [Crossref] [PubMed]
- Tufman AL, Edelmann M, Gamarra F, et al. Preselection based on clinical characteristics in German non-small-cell lung cancer patients screened for EML4-ALK translocation. J Thorac Oncol 2014;9:109. [Crossref] [PubMed]
- Wang Z, Zhang X, Bai H, et al. EML4-ALK rearrangement and its clinical significance in Chinese patients with advanced non-small cell lung cancer. Oncology 2012;83:248-56. [Crossref] [PubMed]
- Corrales-Rodríguez L, Arrieta O, Mas L, et al. An international epidemiological analysis of young patients with non-small cell lung cancer (AduJov-CLICaP). Lung Cancer 2017;113:30-6. [Crossref] [PubMed]
- Yip PY, Yu B, Cooper WA, et al. Patterns of DNA Mutations and ALK Rearrangement in Resected Node Negative Lung Adenocarcinoma. J Thorac Oncol 2013;8:408-14. [Crossref] [PubMed]
- Zhou JX, Yang H, Deng Q, et al. Oncogenic driver mutations in patients with non-small-cell lung cancer at various clinical stages. Ann Oncol 2013;24:1319-25. [Crossref] [PubMed]
- Tanaka K, Hida T, Oya Y, et al. Unique prevalence of oncogenic genetic alterations in young patients with lung adenocarcinoma. Cancer 2017;123:1731. [Crossref] [PubMed]
- Sacher AG, Dahlberg SE, Heng J, et al. Association Between Younger Age and Targetable Genomic Alterations and Prognosis in Non-Small-Cell Lung Cancer. JAMA Oncol 2016;2:313-20. [Crossref] [PubMed]
- Fukui T, Yatabe Y, Kobayashi Y, et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer 2012;77:319-25. [Crossref] [PubMed]
- Liu YT, Shi YK, Hao XZ, et al. Analysis of clinicopathological features of the echinoderm microtubule-associated protein-like-4-anaplastic lymphoma kinase fusion gene in Chinese patients with advanced non-small-cell lung cancer. Thorac Cancer 2014;5:255. [Crossref] [PubMed]
- Kim MH, Shim HS, Kang DR, et al. Clinical and prognostic implications of ALK and ROS1 rearrangements in never-smokers with surgically resected lung adenocarcinoma. Lung Cancer 2014;83:389-95. [Crossref] [PubMed]
- Igata F, Uchino J, Fujita M, et al. Clinical Features of Lung Cancer in Japanese Patients Aged Under 50. Asian Pac J Cancer Prev 2016;17:3377-80. [PubMed]
- Paik JH, Choi CM, Kim H, et al. Clinicopathologic implication of ALK rearrangement in surgically resected lung cancer: a proposal of diagnostic algorithm for ALK-rearranged adenocarcinoma. Lung Cancer 2012;76:403-9. [Crossref] [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [Crossref] [PubMed]
- VandenBussche CJ, Illei PB, Lin MT, et al. Molecular alterations in non-small cell lung carcinomas of the young. Hum Pathol 2014;45:2379-87. [Crossref] [PubMed]
- Subramanian J, Morgensztern D, Goodgame B, et al. Distinctive Characteristics of Non-small Cell Lung Cancer (NSCLC) in the Young: A Surveillance, Epidemiology, and End Results (SEER) Analysis. J Thorac Oncol 2010;5:23-8. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Rosen JE, et al. Lung cancer in the very young: treatment and survival in the National Cancer DataBase. J Thorac Oncol 2016;11:1121-31. [Crossref] [PubMed]
- Zhang J, Chen SF, Zhen Y, et al. Multicenter analysis of lung cancer patients younger than 45 years in Shanghai. Cancer 2010;116:3656. [Crossref] [PubMed]
- Lara MS, Brunson A, Wun T, et al. Predictors of survival for younger patients less than 50 years of age with non-small cell lung cancer (NSCLC): a California Cancer Registry analysis. Lung Cancer 2014;85:264-9. [Crossref] [PubMed]
- Chaft JE, Dagogo-Jack I, Santini FC, et al. Clinical outcomes of patients with resected, early-stage ALK-positive lung cancer. Lung Cancer 2018;122:67-71. [Crossref] [PubMed]
- Kang HJ, Lim HJ, Park JS, et al. Comparison of clinical characteristics between patients with ALK-positive and EGFR-positive lung adenocarcinoma. Respir Med 2014;108:388. [Crossref] [PubMed]
- Gainor JF, Tseng D, Yoda S, et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017;2017. [Crossref] [PubMed]
- Hou H, Zhu H, Zhao H, et al. Comprehensive Molecular Characterization of Young Chinese Patients with Lung Adenocarcinoma Identified a Distinctive Genetic Profile. Oncologist 2018;23:1008-15. [Crossref] [PubMed]


