Challenges in the treatment of early non-small cell lung cancer: what is the standard, what are the challenges and what is the future for radiotherapy?
Introduction
Lung cancer is one of the most common neoplasms, and is a major cause of mortality and morbidity throughout the world; in 2012 there were 410,000 cases and 353,000 deaths in Europe (1). Surgery is the mainstay of treatment for patients with early disease non-small cell lung cancer (NSCLC). For treated patients, the 5-year overall survival (OS) is 50-43% in patients with clinical stage IA-IB, and 73-58% in surgically staged IA-IB patients, respectively (2). Because of medical co-morbidities related to smoke, about 25% of patients with early stage NSCLC do not receive standard surgery, and in case of no treatment, patients with stage IA-IB have a median survival of 17 months (3) according to an observational study.
But there is now an alternative treatment that can give good results. Conventional radiotherapy (RT) was for years the alternative option of early-stage medically inoperable patients, but the results were quite poor (4). Among patients treated with curative intent, the reported 5-year survival rate was 0-42%; 29-37% in T1 and 4-24% in T2N0M0 tumours. It should be outlined that the population eligible for surgery is very different from the population treated with radiotherapy, as the latter had also many co-morbidities. The cause-specific survival (CSS) was 54-93% at 2 years, and 13-19% at 5 years. It was suggested that OS and local control (LC) were affected by tumour size (cut-off 4 cm) and total dose, with better outcome if RT dose was 60-69 Gy (4). Hypofractionated RT has been described as an interesting treatment option for these patients; a dose of 48-52 Gy in 4 Gy fractions could provide LC rates of 70.1% at 5 years (5). Such results are however poor compared to the results observed with stereotactic radiotherapy that was originally developed to treat small intracranial lesions in the 50 s, and then started to be proposed to patients with inoperable early stage lung cancer.
Stereotactic ablative body radiotherapy (SABRT) is defined as an “external beam radiation therapy method used to very precisely deliver a high dose of radiation to an extracranial target within the body, using either a single dose or a small number of fractions” (6). The use of SABRT in lung cancer has been developed in the nineties (7,8) and is nowadays more widely used. It has become over the years, the treatment of choice in inoperable patients and in operable patients who refuse surgery (9).
In this review we sought to explain the role of SABRT in early stage NSCLC, the state of the art, the challenges and the future for this technique.
Present role of SABRT in early stage NSCLC
Results in T1-T2N0M0
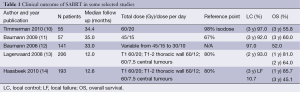
SABRT has become the standard alternative treatment in inoperable patients, due to co-morbidities or age, because of the good results observed (9). In the main published series of SABRT for stage IA-IB NSCLC (Table 1), there is a 3-year LC rate of 90%. Reported early toxicity is quite low, with no treatment-related death in peripheral stage I tumours. The reported acute toxicity (10-14) consists of fatigue (in 31-33% of the patients), local chest pain (in 3-12%) and dyspnea (5-7%). The most frequent late grade III-IV toxicities were pneumonitis (2-3%), thoracic pain (3%) and rib fracture (1-2%).
Full table
Optimal dose
Different SABRT schedules, with different fraction sizes, total doses and modalities of dose prescription have been used making direct comparisons difficult. Some of the published regimens are described in Table 2 (10,13,14). The “Biological Equivalent Dose” or BED may allow easier comparisons of the effects of various treatment protocols (15). A retrospective study has tried to evaluate the optimal SABRT dose by studying the relation between BED and outcome (LC and survival rates) (16). There seems to be a SABRT dose effect, patients treated with higher dose SABRT have lower local recurrence rates: 8.1% if BED ≥100 vs. 26.4% if BED <100 (P<0.01). Within this study, differences in OS were only observed in operable patients treated with SABRT and BED ≥100. Differences in OS are difficult to evaluate, as patients with inoperable NSCLC will eventually die because of severe comorbidities.

Full table
Thus, European Society of Medical Oncology (ESMO) lung cancer guidelines (9) recommend using a SABRT regimen with a BED of ≥100 Gy, delivered to the encompassed isodose (9).
Challenges
What is the optimal dose fractionation?
There is no standard fractionation for SABRT in early stage NSCLC. The most common schedules are listed in Table 2. In a recent overview of SABRT studies (17), as opposed to the multi-institutional Japanese study previously mentioned (16), no relationship was found between LC and total dose suggesting that lower but more uniform doses could be sufficient to get adequate control rates. Another overview divided the studies according to BED in quartiles as “low, medium, medium-high and high” within doses of 51-83, 83-108, 108-145 and 145-196 BED Gy (18). It concluded that there was a tendency of better 2-3-year OS in T1 lesions in the medium BED group, and a better 3-year-OS in the medium-high BED group for T2 tumours. CSS at 3 years was lower in the “low-dose” group.
SABRT for patients with severe pulmonary comorbidities and impact on lung function
In patients with early stage NSCLC with severe chronic ventilatory impairment that undergo surgery (19,20), the postoperative complication rate is quite high (57-70%), with mortality rates of 8-14%. As reported by Lau et al. (20), video-assisted thoracic surgery (VATS) may be particularly interesting for such patients, as it increases 2.8-fold the adjusted OS benefit over the open-standard surgery approach suggesting a decrease of morbidity with VATS compared to classical open surgery. SABRT is an interesting treatment option in patients with severe chronic obstructive pulmonary disease (COPD) (21), achieving LC similar to surgery, with less toxicity (postoperative deaths of 7-25% in the surgery group vs. 0% in SABRT).
The effect of SABRT in terms of its impact on pulmonary function tests (PFT) has been studied in patients included in a radiation therapy oncology group trial (RTOG 0236) by Stanic (22). No significant change in PFT was observed, nor any correlation between PFT decrease and lung toxicity (pneumonitis), or any relationship between decrease of PFT and survival. In a mono-institutional study (23), a low pretreatment forced expiratory volume in 1 second (FEV1) or diffusing capacity of carbon monoxide (DLCO) did not affect the survival of these patients so it was suggested not to refuse SABRT treatment based on a low FEV1 or DLCO.
Regarding to quality of Life in patients treated either with tridimensional radiotherapy (3D-RT) or SABRT (24), only physical function was negatively affected in the 3D-RT group. The authors insisted on the need of new quality of life studies in this group of patients.
SABRT for elderly patients
Concerning SABRT for the elderly (≥75 years), a large published series of 193 patients (14) comparing them to a younger population, found a similar OS and toxicity profile. Another interesting retrospective observational study based on the SEER database in elderly patients with early-stage NSCLC (25) shows that offering a radical treatment to this population may be beneficial. Comparing patients ≥75 years to younger patients (55-74 years), they concluded that the major cause of death in this older population was actually lung cancer. The authors suggest that the use of SABRT in medically inoperable elderly patients should be more frequently considered. Only 1.1% of them received SABRT compared to 14.8% in the younger group. The study of Palma et al. (26) has shown the impact the introduction of SABRT has had, on the outcome of elderly patients in the Netherlands.
Extension of SABRT indications
Larger tumours
In surgically treated patients, tumour size is a well-known prognostic factor. It is not that clear, in SABRT studies, whether outcome varies according to tumour size. In the RTOG 0326 study which included 44 evaluable patients with T1 and 11 patients with T2 tumours treated with 3 fractions of 18 Gy (10), there was no significant difference in terms of median disease-free survival (DFS) between patients with T1 (36.1 months) and T2 tumours (33.7 months).
In the Scandinavian prospective study of Baumann et al. treating 57 patients were treated with 3 fractions of 15 Gy (11), the risk of systemic failure was more frequent in higher stage tumours (T1b-T2 vs. T1a) and in patients with larger tumour volume. In another retrospective study by Baumann et al. (12), the authors studied the factors that could affect the efficacy of SABRT in a series of 138 patients. The authors described a relationship between local failure rate, stage and gross tumour volume (GTV): in T1 tumours, there were less local failures (3%) than in T2 tumours (13%). GTV volume (<26 cm3) was also related to a decreased local failure rate. No differences in survival or metastatic rates were found between T1 and T2 tumours in this study. The crude distant metastases failure rate was 25%.
In a multi-institutional Japanese study published by Onishi et al. (16), there was a higher local recurrence rate in stage IB tumours compared to IA in patients treated with a BED <100 Gy (41.4% vs. 16.6%). Such difference was not observed in patients who were treated with a BED >100 Gy.
In the systematic review by Chi et al. (27) LC was poorer in tumours larger than 5 cm, especially if a BED of <100-120 Gy was administered. For this reason they suggest using higher doses for larger tumours (BED above 120 Gy). It should be underscored that few studies have included patients with tumours over 5 cm. In studies that included patients with tumours over 5 cm, the reported risk of distant metastases approached 30%.
Interestingly, in the recently published Princess Margaret Hospital experience of 185 T1-T2N0 patients treated with SABRT (28), tumour size was not related to local failure whereas regional and distant failure rate as well as OS, DFS and CSS were related to T size. The prescribed dose schedule was risk-adapted based on tumour size and location. They concluded that SABRT was a good therapeutic option and could be proposed in larger tumours up to 5.7 cm in diameter or 100 cc. However they state that larger tumours seem to be associated with more non-local failures so that more extensive staging and adjuvant treatment should be considered.
Based on these retrospective studies, it seems that if a BED over 100 Gy is used, in tumours of 5 cm or less, there may be no difference in terms of LC. However tumour size seems to impact more on the risk of regional or distant failure.
Central early lung cancer
The main limitation to SABRT in centrally located tumours is due to its potential severe toxicity. This has been described by Timmerman (29) in a prospective phase II study that included 70 patients treated with SABRT (60-66 Gy in 3 fractions) whatever the tumour location. Although an excellent LC was observed (95% actuarial at 2 years), the authors reported increased grade III-V toxicity. They described an 11-fold probability of grade III-IV toxicity in central locations as compared with peripheral tumours: at 2 years, 83% of patients with peripheral tumours were free from severe toxicity as opposed to 54% of patients with central tumours. Tumour size of >10 mL was also associated with a higher risk of toxicity. The authors concluded that SABRT in tumours located within 2 cm of the proximal bronchial tree was at high risk of toxicity, so that this regimen (60 Gy in 3 fractions) should not be used in centrally located tumours. Since then, other authors have shown that such severe toxicities were rare if more fractionated regimens were used in central tumours defined as tumours localized within 2 cm of the proximal bronchial tree (13,29).
In a review concerning central tumours treated with SABRT, Senthi et al. (30) concluded that a good LC (>85%) could be achieved if BED10 ≥100 Gy, as seen in peripheral tumours. Treatment-related mortality was 2.7%, so higher than that observed in peripheral tumours. So, such results could be achieved with fractionation schedules of 50 Gy in 10 Gy-fractions, 54 Gy in 9 Gy fractions, 56 Gy in 8 Gy fractions or 60 Gy in 7.5 Gy fractions. A grade III-IV toxicity was present in <9% of patients. Local, regional or distant control was not affected by tumour location (central vs. peripheral) if a BED10 ≥100 Gy was administered.
It should be outlined that bronchial stenosis is a very rare complication in patients treated with 3-D conformal radiotherapy at doses of 60-66 Gy. However, there is an increased risk when higher doses are used as shown in a study by Miller et al. (31). These authors found a 4% risk stenosis among patients treated with 74 Gy, and a higher risk (25%) among patients who had received 86 Gy. A grade V toxicity occurred in 3% of the patients, of whom one developed a broncho-pleural fistula.
Toxicities observed and implications for risk-adapted SABRT
The concept of risk-adapted fractionated stereotactic radiotherapy was developed, to insure good results and avoid severe toxicities, depending upon location and tumour size (13,28). A study comparing 2 fractionation regimes (32) (60 Gy delivered in 3 fractions vs. 50 Gy delivered in 5 fractions) showed an increased grade I-II chronic chest wall toxicity in the 60 Gy regime (18%) compared to the 50 Gy regime (4%) suggesting this second fractionation was preferable for larger lesions close to the chest wall. In the present time dose and fractionation is adapted according to tumour location (central vs. peripheral), proximity to the chest wall (more or less than 1 cm) and tumour size.
Several articles have been published recently focusing on clinical factors potentially related with chest wall toxicity secondary to SABRT (33-36). Chest wall pain can be quite frequent (around 20-25%) when tumours are close to chest wall, especially in large tumours. The incidence of rib fractures may vary between 1.6% and 23% in these articles. Rib fractures cause chest wall pain, but in about in 1/3 patients, they are asymptomatic. Even if several parameters have been proposed, there are no clear and consensual predictive volumetric data for chest wall grade III-IV toxicity because of the low incidence of such events. Most authors propose to lower the dose per fraction in case of larger tumours close to chest wall or in smaller tumours adherent to chest wall.
There are presently multicentre studies evaluating SABRT in central tumours, with more fractionated regimens compared to the prospective RTOG 0236 study (29,37,38). The Lung Tech European Organisation for Research and Treatment of Cancer (EORTC) trial (37) (NCT01795521) is a phase II trial evaluating both the efficacy and toxicity of a risk-adapted SABRT regimen (60 Gy in 8 fractions) within a multicentric setting in medically inoperable patients. The RTOG 0813 trial (38) is a phase I/II multicentric trial, which is also specifically addressing the issue of the optimal dose for central tumours. According to the results and possible toxicity observed, dose will be either increased or decreased by 0.5 Gy per fraction, starting at a dose of 50 Gy in 10 Gy-fractions. In any case, results of prospective studies are needed in order to establish the optimal dose-fractionation schedule. Long-term follow-up is very important.
It is recommended to delineate carefully the target volume and organs at risk, to decide the optimal treatment plan on a 4-D CT (39,40), after staging evaluation including a recent PET-CT and thoracic CT-scan.
Difficulties in assessing local control
Assessing LC may be quite challenging, as radiologic changes after SABRT are difficult to distinguish from local recurrence. Patients should be followed up, as there are radiological changes in all patients. Several types of changes have been described that evolve throughout time as well described by the Amsterdam Free University team (23,41,42) and Guckenberger (43). According to Dahele et al. (42), median time of onset for radiologic changes was 17 weeks. The percentage of patients who developed radiological changes was 54% at 6 months, 73% at 12 months and 87% at 24 months (44). The most common late CT changes were: modified pattern of fibrosis (71%), scar-like fibrosis (11%) and “mass-like” fibrosis that can be tricky. The highest severity of radiological changes has been described at 1-2 years, tending to decrease afterwards. A rapidly growing mass after SABRT could be indicative of a real local recurrence. Mattonen et al. (45) propose a quantitative analysis of CT-scan changes of tumour lesions based on 3D-volume, T size according to RECIST criteria changes of Hounsfield Units, ground glass opacity textural analysis, to differentiate benign Radiation-induced lung injury (RILI) from recurrence, as early as possible.
Patterns of failure after SABRT
As said previously, the local recurrence rate is about 10% at 3 years in most studies. Patients with an adequate pretreatment study (PET-CT) have a regional failure of 10%, and distant failure rate seems higher, depending upon stage. Concerning regional failure, Hoopes (44) studied a cohort of 58 patients, who all had a 18-FDG PET-CT before SABRT for an early-stage NSCLC. Median follow up was 42.5 months. The authors describe a risk of nodal failure of 25%. Isolated nodal failure was found in 6 patients (10%). However interpretation of PET-CT can sometimes be difficult: a metabolic activity in the treated volume could be found in 7% patients with a SUV of 2.5-5.9, and with no local recurrence proven.
Patterns of failure were well described in a large retrospective cohort study of 676 patients with early-stage NSCLC by Senthi et al. (46). All patients had a pre-treatment FDG-PET-CT. With a median follow-up of 32.9 months, 4% patients presented a local recurrence at a median time of onset of 15 months. The regional recurrence rate was 6% with a median time of onset of 13 months, whereas the distant failure rate was 12%, and the median time of onset of 9.6 months. Isolated loco-regional recurrence (and hence potentially resectable patients) represented 34% of all recurrences, of whom only 31% were medically operable. Most common site of distant recurrence was contralateral lung. Among the 42 patients with initial loco-regional recurrence, 17% developed distant metastases at a median time of 9 months. Second primary cancer appeared in 6% of patients at a median time of 18 months, most frequent located in contralateral lung.
Results in operable patients according to size
The patterns of recurrence are quite similar in operable patients treated with stereotactic radiotherapy. Onishi (47) published a series of 87 T1-2N0M0 NSCLC medically operable patients, with a median follow-up of 55 months. The risk of local, nodal and distant recurrence was 9.2%, 15%, and 22%, respectively. LC at 5 years was significantly better in patients with stage IA (92%) than stage IB (73%). However, there was no difference according to stage regarding regional or distant control or OS.
Any role for adjuvant chemotherapy in fit patients treated with SABRT?
As distant failure seems more frequent (20-25% of patients) than local or mediastinal failure, there may be some rationale then to envisage adjuvant chemotherapy in fit patients. The possible role of “adjuvant chemotherapy” has been questioned by a small study by Chen et al. (48). They published a series of 65 T1-3N0M0 NSCLC patients treated with SABRT at a BED of 115-72 Gy with or without chemotherapy according to medical co-morbidities. Chemotherapy was a platinum-based regime. The study concluded that adjuvant chemotherapy improved 5-year OS by 14%. No conclusion can be drawn from such a study, as only fit patients could have adjuvant chemotherapy, but it shows it should be further evaluated, if indications of SABRT extend to operable patients.
Series comparing surgery-SABRT
As surgery is the gold-standard treatment for early stage NSCLC, and as the accumulated evidence in favour of SABRT in inoperable patients increases, there has been several studies (overviews and a few matched-pair analyses) trying to compare surgery and SABRT. Onishi was the first one to address this issue, in a series of 87 operable early stage NSCLC patients (47). With a median follow-up of 55 months, LC at 5 years was 87%, regional control (lymph node metastasis) was 85.3% and distant metastasis control was 75%. Five-year OS and CSS was 70% and 76% respectively. Even when they compared these results to surgical series that are, much larger and with longer follow-up times, they concluded that the results of SABRT could be potentially equivalent to surgery.
Grills et al. (49) published a comparative retrospective cohort of stage I NSCLC patients treated with either wedge resection (n=58) or SABRT (n=69, of whom 95% were inoperable). Outcomes were similar in both groups. OS was better in the surgery group, but CSS was similar; probably, because SABRT group was composed mainly of inoperable patients, and that patients will eventually succumb of non-cancer related causes.
In a systematic review, Soldà et al. (50), compared the 2-year OS of stage I patients who were treated either with surgery (2,038 patients) or SABRT (3,201 patients). Results were similar: 70% for SABRT (95% CI: 67-72%) and 68% for surgery (95% CI: 66-70%). The LC rate at 2 years for patients treated with SABRT was 91% (95% CI: 90-93%). Patients who had SABRT were treated with different available technologies, and the authors could not find any difference in terms of outcome or OS.
A propensity-score matched analysis was published by Verstegen et al. (51), matching T1-3N0M0 NSCLC treated with surgery (VATS or lobectomy 64 patients) or SABRT (64 patients). Median follow up was 16 and 30 months respectively. Unsuspected nodal disease was found in 19% of VATS patients. Locorregional control (LRC) was better in SABRT group (1-3-year LCR 97-94%) than in VATS group (1-3-year LCR 87-83%), with a hazard ratio of 3.68, 95% CI: 1.09-12.50, P=0.04. Distant recurrence rate, OS and Freedom from Progression was similar in both groups. Median time to recurrence was 11 months in the SABRT group and 8.2 months in the surgery group.
Two retrospective series of the same team comparing SABRT and surgery by Crabtree and Robinson are also interesting. They underline that nodal dissection may show unforeseen nodal involvement in up to 37% of patients operated for clinical stage I NSCLC (52,53). In a series of patients treated either by surgery (458 patients) or SABRT (151 patients), they showed a regional (N1-N2) upstaging of 15% of the patients from the surgery group. Three-year OS of patients with occult nodal disease was obviously worse (66%) compared to those without occult nodal disease (80%). The median follow up was 2.83 years in the surgical series and 1.95 years in the SABRT group. After a matched comparison according to age, tumour size, tumour location, FEV1 and comorbidities (56 matched patients in each cohort) it was concluded that 3-year OS was better in surgical group (68%) vs. SABRT (52%). Disease-free survival was 65% vs. 47%, respectively, and 3-year local recurrence-free survival was 91% and 92%, respectively, but not statistically significant.
Such studies provide interesting information, but drawing conclusions from retrospective studies is hazardous. There are few data on long-term outcome after SABRT, and SABRT is a much “younger” treatment than surgery. Randomized trials would be needed to really compare these two treatments. There have been 3 unfortunate attempts to run randomized trials comparing SABRT to surgery that have closed due to poor accrual (54-56). Guidelines have been published about SABRT implementation to propose clinical trials (57).
Conformal Conventional Radiotherapy for early stage NSCLC
Not all inoperable patients with early lung cancer can be treated with SABRT, and patients especially those with N1 involvement or patients with larger tumours (over 5-7 cm) may be still treated with conventional conformal radiotherapy. In a systematic review of Rowell et al. the results of conventional RT for patients with early stage NSCLC were quite mediocre (4). In T1 and T2 tumours, the 5-year survival rate was 29-37%, and 4-24% respectively. Local recurrence rate ranged between 6% and 70% showing the difficulty of LC assessment. As expected, tumour size, nodal involvement, age over 70 years, presence of comorbidities and weight loss, were all factors that impacted negatively on outcome. An improved survival was observed in patients less than 70 years old, higher delivered doses or squamous carcinoma histology.
Altered fractionation has been evaluated, and showed very promising results in the continuous hyperfractioned accelerated radiotherapy (CHART) landmark randomised study (58,59) comparing CHART (54 Gy in 36 fractions and 12 consecutive days with 3 fractions per day) to conventional RT (60 Gy in 30 daily fractions, 5 days per week); 36% of patients had stage I and II NSCLC. The outcome was significantly improved among patients who had accelerated RT, with a 22% of reduction of the relative risk of death. The 2-year OS was 30% in hyperfractionated group against 21% in the control group. However, even if the results are quite interesting, this schedule has not become standard treatment, mostly because of organizational issues. More recently, an individual data-based meta-analysis has validated this approach (60).
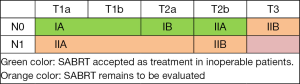
SABRT is nowadays accepted as the treatment for medically inoperable cT1-2N0M0 NSCLC patients (9). In patients with cT1-2N1M0, or T3N0M0, conventional RT is still considered the standard in inoperable patients, but the interesting results observed with altered fractionation should be highlighted. SABRT remains to be evaluated in larger tumours; the role of SABRT in centrally located tumours is under study (Figure 1: green color: SABRT accepted as treatment; orange color: SABRT remains to be evaluated).
Future of RT in early NSCLC
More prospective studies with defined endpoints and follow-up evaluation are needed in peripheral tumours as well as centrally located tumours. We do not yet know, whether SABRT may have a role in operable patients. As the number of fit patients undergoing SABRT may increase in the future, it will probably lead to better knowledge of long-term outcome. Follow-up of long-term survivors will become of outmost importance, since most patients treated with SABRT up to now, had severe co-morbidities, and would eventually die of non-cancer related causes. More extensive exploration of the mediastinum will probably be needed. Adjuvant treatments will then have to be evaluated.
In the future, individualized treatment in terms of dose and fractionation, may vary according to molecular, radiomics and radiosensitivity profile.
Lung cancer screening represents another challenge. At present, most NSCLC are diagnosed at an advanced stage. The recently reported results of screening trials in a high-risk population are quite provocative for thoracic oncologists. The National Lung Screening Trial (NLST) comparing chest X-ray to low dose CT (61,62), has shown a relative reduction of death from lung cancer of 20% in the low dose CT group. Screening of lung cancer promotes an earlier diagnosis of the disease, allowing the onset of more radical treatments. SABRT may have an important role as an alternative to surgery. In a review about treatment options in early stage lung cancer (surgery or SABRT) (63), authors conclude that operable patients should be operated (lobectomy) with the advantage of having complete pathological results. For unfit patients, SABRT should be offered as the alternative treatment with the main advantage of its low toxicity. For intermediate-fit patients they propose to encourage clinical trials to establish indications of these two treatment modalities, so that we can better individualize the optimal treatment for every single patient.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Vrdoljak E, Mise K, Sapunar D, et al. Survival Analysis of Untreated Patients With Non-Small-Cell Lung Cancer. Chest 1994;106:1797-800. [PubMed]
- Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable): a systematic review. Thorax 2001;56:628-38. [PubMed]
- Soliman H, Cheung P, Yeung L, et al. Accelerated hypofractionated radiotherapy for early-stage non-small-cell lung cancer: long-term results. Int J Radiat Oncol Biol Phys 2011;79:459-65. [PubMed]
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [PubMed]
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [PubMed]
- Uematsu M, Shioda A, Tahara K, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer 1998;82:1062-70. [PubMed]
- Vansteenkiste J, Crinò L, Dooms C, et al. 2nd ESMO Consensus Conference on Lung Cancer: early-stage non-small-cell lung cancer consensus on diagnosis, treatment and follow-up. Ann Oncol 2014;25:1462-74. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA 2010;303:1070-6. [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a Prospective Phase II Trial of Medically Inoperable Stage I Non–Small-Cell Lung Cancer Patients Treated With Stereotactic Body Radiotherapy. J Clin Oncol 2009;27:3290-6. [PubMed]
- Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncologica 2006;45:787-95. [PubMed]
- Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685-92. [PubMed]
- Haasbeek CJ, Lagerwaard FJ, Antonisse ME, et al. Stage I Nonsmall Cell Lung Cancer in Patients Aged >75 Years. Outcomes After Stereotactic Radiotherapy. Cancer 2010;116:406-14. [PubMed]
- Yaes RJ, Patel P, Maruyama Y. On using the linear-quadratic model in daily clinical practice. Int J Radiat Oncol Biol Phys 1991;20:1353-62. [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic Hypofractionated High-Dose Irradiation for Stage I Non Small Cell Lung Carcinoma Clinical Outcomes in 245 Subjects in a Japanese Multiinstitutional Study. Cancer 2004;101:1623-31. [PubMed]
- van Baardwijk A, Tomé WA, van Elmpt W, et al. Is high-dose stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer (NSCLC) overkill? A systematic review. Radiother Oncol 2012;105:145-9. [PubMed]
- Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2011;81:e305-16. [PubMed]
- Magdeleinat P, Seguin A, Alifano M, et al. Early and long-term results of lung resection for non-small-cell lung cancer in patients with severe ventilatory impairment. Eur J Cardiothorac Surg 2005;27:1099-105. [PubMed]
- Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient-the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13. [PubMed]
- Palma D, Lagerwaard F, Rodrigues G, et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systematic review. Int J Radiat Oncol Biol Phys 2012;82:1149-56. [PubMed]
- Stanic S, Paulus R, Timmerman RD, et al. No Clinically Significant Changes in Pulmonary Function Following Stereotactic Body Radiation Therapy for Early- Stage Peripheral Non-Small Cell Lung Cancer: An Analysis of RTOG 0236. Int J Radiat Oncol Biol Phys 2014;88:1092-9. [PubMed]
- Henderson M, McGarry R, Yiannoutsos C, et al. Baseline pulmonary function as a predictor for survival and decline in pulmonary function over time in patients undergoing stereotactic body radiotherapy for the treatment of stage I non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:404-9. [PubMed]
- Widder J, Postmus D, Ubbels YJ, et al. Survival and quality of life after stereotactic or 3d-conformal radiotherapy for inoperable early-stage lung cancer. Int J Radiat Oncol Biol Phys 2011;81:e291-7. [PubMed]
- Varlotto JM, Decamp MM, Flickinger JC, et al. Would screening for lung cancer benefit 75- to 84-year-old residents of the United States? Front Oncol 2014;4:37. [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small-cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153-9. [PubMed]
- Chi A, Liao Z, Nguyen NP, et al. Systemic review of the patterns of failure following stereotactic body radiation therapy in early-stage non-small-cell lung cancer: Clinical implications. Radiother Oncol 2010;94:1-11. [PubMed]
- Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;87:1064-70. [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive Toxicity When Treating Central Tumors in a Phase II Study of Stereotactic Body Radiation Therapy for Medically Inoperable Early-Stage Lung Cancer. J Clin Oncol 2006;24:4833-9. [PubMed]
- Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013;106:276-82. [PubMed]
- Miller KL, Shafman TD, Anscher MS, et al. Bronchial stenosis: an underreported complication of high-dose external beam radiotherapy for lung cancer? Int J Radiat Oncol Biol Phys 2005;61:64-9. [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. A Comparison of Two Stereotactic Body Radiation Fractionation Schedules for Medically Inoperable Stage I Non-small Cell Lung Cancer. The Cleveland Clinic Experience. J Thorac Oncol 2009;4:976-82. [PubMed]
- Andolino DL, Forquer JA, Henderson MA, et al. Chest wall toxicity after stereotactic body radiotherapy for malignant lesions of the lung and liver. Int J Radiat Oncol Biol Phys 2011;80:692-7. [PubMed]
- Bongers EM, Haasbeek CJ, Lagerwaard FJ, et al. Incidence and Risk Factors for Chest Wall Toxicity after Risk-Adapted Stereotactic Radiotherapy for Early-Stage Lung Cancer. J Thorac Oncol 2011;6:2052-7. [PubMed]
- Nambu A, Onishi H, Aoki S, et al. Rib fracture after stereotactic radiotherapy for primary lung cancer: prevalence, degree of clinical symptoms, and risk factors. BMC Cancer 2013;13:68-75. [PubMed]
- Pettersson N, Nyman J, Johansson KA. Radiation-induced rib fractures after hypofractionated stereotactic body radiation therapy of non-small cell lung cancer: A dose-and volume-response analysis. Radiother Oncol 2009;91:360-8. [PubMed]
- Nestlé U, Collette S, Meulders I, et al. LungTech Stereotactic Body Radiotherapy (SBRT) of Inoperable Centrally Located NSCLC: A Phase II Study in Preparation for a Randomized Phase III Trial. Available online: http://clinicaltrials.gov/show/NCT01795521 http://www.eortc.org/clinical-trials
- Bezjak A, Bradley J, Gaspar L, et al. RTOG 0813: Seamless Phase I/II study of Stereotactic Lung Radiotherapy (SBRT) for early stage, centrally located, Non-Small Cell Lung Cancer (NSCLC) in medically inoperable patients. Available online: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0813 http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?action=openFile&FileID=9067
- De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol 2010;28:5301-10. [PubMed]
- Kong FM, Ritter T, Quint DJ, et al. Consideration of dose limits for organs at risk of thoracic radiotherapy: atlas for lung, proximal bronchial tree, esophagus, spinal cord, ribs, and brachial plexus. Int J Radiat Oncol Biol Phys 2011;81:1442-57. [PubMed]
- Dahele M, Senan S. The Role of Stereotactic Ablative Radiotherapy for Early- Stage and Oligometastatic Non-small Cell Lung Cancer: Evidence for Changing Paradigms. Cancer Res Treat 2011;43:75-82. [PubMed]
- Dahele M, Palma D, Lagerwaard F, et al. Radiological Changes After Stereotactic Radiotherapy for Stage I Lung Cancer. J Thorac Oncol 2011;6:1221-8. [PubMed]
- Guckenberger M, Meyer J, Wilbert J, et al. Intra-fractional uncertainties in cone-beam CT based image-guided radiotherapy (IGRT) of pulmonary tumors. Radiother Oncol 2007;83:57-64. [PubMed]
- Hoopes DJ, Tann M, Fletcher JW, et al. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 2007;56:229-34. [PubMed]
- Mattonen SA, Palma DA, Haasbeek CJ, et al. Distinguishing radiation fibrosis from tumour recurrence after stereotactic ablative radiotherapy (SABR) for lung cancer: A quantitative analysis of CT density changes. Acta Oncologica 2013;52:910-8. [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [PubMed]
- Chen Y, Guo W, Lu Y, et al. Dose-individualized stereotactic body radiotherapy for T1–3N0 non-small cell lung cancer: Long-term results and efficacy of adjuvant chemotherapy. Radiother Oncol 2008;88:351-8. [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [PubMed]
- Soldà F, Lodge M, Ashley S, et al. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; Systematic review and comparison with a surgical cohort. Radiother Oncol 2013;109:1-7. [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [PubMed]
- Crabtree TD, Puri V, Robinson C, et al. Analysis of first recurrence and survival in patients with stage I non-small cell lung cancer treated with surgical resection or stereotactic radiation therapy. J Thorac Cardiovasc Surg 2014;147:1183-91; discussion 1191-2. [PubMed]
- Robinson CG, DeWees TA, El Naqa IM, et al. Patterns of failure after stereotactic body radiation therapy or lobar resection for clinical stage I non-small-cell lung cancer. J Thorac Oncol 2013;8:192-201. [PubMed]
- Available on: http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1021
- Available on: http://clinicaltrials.gov/ct2/show/nct00687986
- Available on: http://clinicaltrials.gov/show/NCT00840749
- Hurkmans CW, Cuijpers JP, Lagerwaard FJ, et al. Recommendations for implementing stereotactic radiotherapy in peripheral stage IA non-small cell lung cancer: report from the Quality Assurance Working Party of the randomised phase III ROSEL study. Radiation Oncology 2009;4:1-14. [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous hyperfractionated accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small-cell lung cancer: a randomised multicentre trial. Lancet 1997;350:161-65. [PubMed]
- Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: mature data from the randomised multicentre trial. Radiotherapy and Oncology 1999;52:137-148. [PubMed]
- Mauguen A, Le Péchoux C, Saunders MI, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol 2012;30:2788-97. [PubMed]
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [PubMed]
- Senan S, Paul MA, Lagerwaard FJ. Treatment of early-stage lung cancer detected by screening: surgery or stereotactic ablative radiotherapy? Lancet Oncol 2013;14:e270-74. [PubMed]