The first attempt worldwide to test PD-1 antibody on multiple “Stage 0” lung cancer
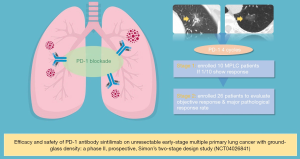
The editorial board recently noticed that a Chinese research team had launched a phase II, prospective, Simon’s two-stage design study which evaluates the efficacy and safety of PD-1 antibody Sintilimab on unresectable early-stage multiple primary lung cancer (MPLC) with ground-glass density (CCTC-1901, NCT04026841) (Figure 1). This is the first documented study worldwide to test PD-1 antibody on “Stage 0” lung cancer, on July 24, 2019.
The incidence of MPLC, along with lung cancer, has increased rapidly in recent years, with an estimated 5% among all of lung cancer cases. Most of the lesions being found in MPLC are in the early stage; therefore, every single lesion is resettable. However, due to the widespread nature of lesions and the risk of further secondary primary lesions, complete resection is not always available. There is not yet a standard treatment for MPLC; however, there are various strategies, such as multiple resections, an intense follow-up, or even lung transplantation. “Those patients, who have multiple lesions or have residual lesions after removing the major ones, are extremely anxious. We want to find a way to help them”, said by Jianxing He, the principal investigator (PI) of this study, who expects that the immunotherapy including PD-1 antibody can play a role in treating MPLC.
PD-1 blockade has become the standard of care in metastatic lung cancer and has been shown to have excellent treatment potency in the earlier stage, e.g., serving as a maintenance treatment after curative chemo-radiotherapy for locally advanced lung cancer, or induction treatment before surgery for resectable lung cancer. Wenhua Liang, the co-PI and designer of this study, told the editors that “some investigators believe that immunotherapy will deliver greater benefits when cancer has not prevailed over the immune system, e.g., in the early stage, and there is some clinical evidence that supports this. However, it remains unknown whether the PD-1 antibody affects early-stage lung cancer, especially the ground-glass lesions, which are predominantly non-invasive or minimally invasive in pathology and are usually regarded as ‘stage 0’ disease. The pros are that PD-L1 directed immune evasion has existed in the early-stage even in pre-invasive stage and PD-1 antibody has a chance to eradicate any cancer before there is significant immune dysregulation; the cons are the low immunogenicity at an early stage and the uncertain risk from immune toxicities. Therefore, we designed this study to answer this controversial and interesting question.”
This study is led by the Chinese clinical trial consortium (CCTC) and is approved and executed by the ethical committee of the first affiliated hospital of Guangzhou medical university (China national clinical research center of respiratory disease). Simon’s optimal two-stage design is used to evaluate the response of PD-1 antibody for multiple lung cancer with ground-glass density. All included patients have a pathological diagnosis of lung cancer, and the lesion evaluated were diagnosed by pathology or by a multidisciplinary team’s consensus. The enrolled patients will receive up to 4 cycles of intravenous infused Sintilimab 200 mg every 3 weeks. The primary endpoint is objective response rate (ORR) evaluated by RECIST 1.1 and secondary endpoints are major pathological response rate (mPR) and safety. It is required that at least one out of 10 enrolled patients in the first stage show objective response before starting the second stage to enrollment another 19 patients. An ORR of 20% is expected to confirm the positive result, considering no more than 5% spontaneous remission during nature course. Also, the investigators will monitor the immune biomarkers [T/B/natural killer (NK) subpopulation, T-cell receptor (TCR) repertoire, exosomal T cell gene expression profile, etc.] during treatment to validate the immune activity.
Acknowledgments
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.