Effective targeted therapy based on dynamic monitoring of gene mutations in non-small cell lung cancer
Introduction
Lung cancer is still the malignant tumor type with the highest morbidity and mortality in the world (1). In recent years, with the development of oncomolecularbiology and the emergence of the concept of precision medicine, several scholars have attempted to provide individualized treatment for breast cancer patients. Molecular targeted therapy, as a new method of tumor treatment different from the conventional treatment methods of malignant tumor, such as surgery, chemotherapy, and radiotherapy, has become an intensely researched area of tumor treatment with its advantages of high efficacy, low toxic side effects, and high specificity for patients with epidermal growth factor receptor (EGFR)-mutant lung cancer. EGFR has tyrosine kinase activity and is associated with the proliferation, angiogenesis, invasion, and metastasis of tumor cells (2). EGFR-tyrosine kinase inhibitors (EGFR-TKIs) can reversibly compete with adenosine triphosphate-binding sites of EGFR, and block EGFR signal transduction, thereby inhibiting the growth and proliferation of tumor cells (3). Several clinical studies (4-6) have confirmed that EGFR-TKI was superior to chemotherapy alone in the treatment of advanced non-small cell lung cancer (NSCLC) with EGFR mutation. In addition, overall response rate (ORR) was estimated to be within 70%, and was shown to significantly prolong progression-free survival (PFS) and overall survival (OS) of patients (7). EGFR-TKIs have been recommended by the National Comprehensive Cancer Network (NCCN) as a potential first-line treatment for advanced NSCLC patients positive for EGFR mutations (8).
About 90% of EGFR mutations are 19 deletions or L858R point mutations. In the majority of cases, EGFR mutation does not simultaneously occur with other carcinogenic gene mutations (such as KRAS mutation or ALK fusion). In the actual treatment, the majority of patients who are sensitive to EGFR-TKI therapy develop drug resistance in about 12 months. The most common mechanism of acquired resistance is the EGFR T790M gatekeeper mutation which is detectable in approximately 50–60% of patients; the other drug resistance mechanisms include C-Met, HER-2 amplification (15–20%), and others (9).
Osimertinib treatment is effective in patients with T790M mutation, and drug resistance mainly occurs after a period of time. A cohort study used next generation sequencing (NGS) on 143 patients and revealed that 41 patients developed drug resistance. Additionally, T790M mutation was detected in 13 patients, and EGFR C797S mutation was detected in 9 patients. Among 28 individuals with loss of T790M, a range of competing resistance mechanisms was detected, including RET, FGFR3, BRAF fusion, and KRAS mutations (10). With the broad application of osimertinib in clinical practice, acquired drug resistance has become the main reason for the failure of treatment with EGFR-TKIs (11-17). Investigations of the causes of this drug resistance from molecular and clinical perspectives are of great importance in overcoming this problem. Genomic analysis of cell-free DNA in plasma has been used to explain two new drug resistance mechanisms of osimertinib: acquired EGFR C797S mutation and T790M mutation deletion (18). In this study, we report a case of lung adenocarcinoma with T790M mutation deletion and subsequent MET amplification as the mechanism of EGFR-TKI resistance.
Case presentation
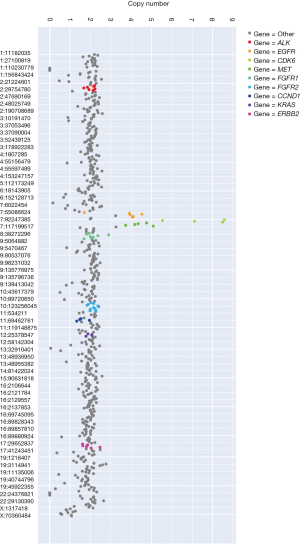
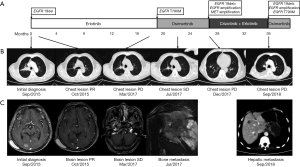
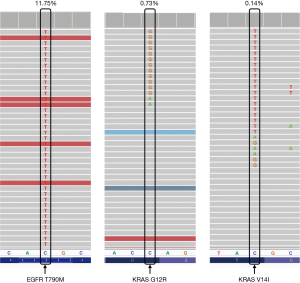
A 64-year-old Chinese Han male was admitted to our hospital on September 1st, 2015 for vertigo and fatigue lasting more than half a month, along with cough and expectoration for 2 days. He was diagnosed with adenocarcinoma of the right lung by transbronchial biopsy, accompanied by bone metastasis and intracranial metastasis. Tissue detection of EGFR gene indicated deletion of exon 19 (Figure 1A) by real-time PCR. Erlotinib was taken orally at 150 mg capsule per day and zoledronic acid intravenous drip was used for bone treatment beginning September 7th, 2015. On October 14th, the results of thoracic and abdominal computed tomography (CT) scanning showed the primary lesion was smaller than before (Figure 1B). Magnetic resonance imaging (MRI) of the brain revealed that the metastasis was significantly smaller than before, and the surrounding edema was reduced (Figure 1C). The curative effect was evaluated as partial remission (PR). After that, the patient complained of dizziness that was aggravated, and was subsequently treated with whole brain radiotherapy PTV 30 Gy/10 F. In September 2016, the MRI reexamination of the brain showed that the brain lesion was smaller and the surrounding edema had disappeared. However, the thoracic lesions progressed, and the plasma-derived circulating tumor DNA (ctDNA) detection of T790M in EGFR gene indicated T790M mutation by targeted NGS, the frequency of which was 1.7%. Since osimertinib has not yet been listed in China, chest radiotherapy PTV 60 Gy/30 F was then successfully performed, and treatment with erlotinib continued. On the March 16th, 2017, chest-abdomen-pelvis CT showed progression of the disease (Figure 1B), and MRI of the brain showed stable disease (SD) of the lesion (Figure 1C). Osimertinib was taken orally from April 25th, 2017 (Figure 1A). On the July 24th, 2017, chest-abdomen-pelvis CT showed the lesion as SD (Figure 1B), but the pain in the left shoulder had worsened. The MRI examination of the left shoulder showed metastatic tumor at the left scapula (Figure 1C). From August 1st, 2017 to September 8th, 2017, intensity-modulated radiation therapy (IMRT) was carried out by 6-MX X-ray on the left shoulder joints, and the pain was relieved. On December 5th, 2017, chest-abdomen-pelvis CT showed that the main lesion of the right lung was stable, and new lesions appeared in the right lung and the lower lobe of the left lung, indicating progressive disease (PD) (Figure 1B). EGFR exon 19 p.L747_P753 delinsS (26.99%), MET amplification (4), and EGFR amplification (4) were found by targeted NGS of ctDNA (Figure 2). Targeted therapy with crizotinib plus erlotinib was started. In August 2018, chest CT scanning showed pleural effusion (Figure 1B), abdominal CT scanning showed hepatic metastasis (Figure 1C), suggesting PD. Panel detection of cancer driver gene revealed EGFR exon 19 p.L747_P753 delinsS (85.78%), exon 20 p.T790M (11.75%), EGFR amplification (4), KRAS exon 2 G12R (0.73%), and KRAS exon V14I (0.14%) by targeted NGS of ctDNA (Figure 3). At present, the patient is under treatment with osimertinib.
Discussion
Selection of molecular targeted drugs according to different molecular characteristics can significantly prolong the median survival time of patients. Kris et al. (19) conducted a retrospective analysis on 733 patients with advanced NSCLC, and found that the maximum median survival time for patients who had a gene mutation that could be targeted by drugs and receive the corresponding targeted therapy was 3.49 years, which was significantly higher than that of the other two groups. Moreover, targeted therapy, similar to chemotherapy, also faces the drug resistance problems and therapeutic approaches should be further studied to find out new targets. The use of various targeted drugs, the continuous monitoring of drug resistance in treatment process, and advancing targeted therapies all depend on the wide application of NGS in clinical practice.
In this study, a typical case of EGFR-mutation-positive pulmonary adenocarcinoma combined with brain metastasis was reviewed. The drug resistance was dynamically monitored in peripheral blood during the treatment, the targeted therapy was carried out, and sustained remission was eventually achieved. Initially, EGFR gene detection indicated a typical exon 19 deletion, and the disease was controlled after treatment with erlotinib (PFS was 12 months). Afterwards, the disease progressed, reflecting resistance to erlotinib, the genetic testing results showed T790M mutation, and erlotinib continued to be taken orally for 5 months after local treatment. Subsequently, the patient was treated with osimertinib for continuous targeted therapy (PFS was 8 months). After 28 months of treatment, the disease progressed again. The NGS results showed that in addition to EGFR 19del+, EGFR gene copy number and MET gene copy number were both amplified. Then, crizotinib plus gefitinib therapy was undertaken. After 36 months of treatment, the NGS results showed a T790M mutation; thus, the patient was alternatively treated with osimertinib.
Compared with the traditional detection methods, the sensitivity of NGS is a remarkable advancement, with a reported detection sensitivity of 0.1–1.0%. Increased sensitivity has made it possible to detect ctDNA in blood samples and has enabled the analysis of fusion genes and copy number variations. Additionally, the detection method of ctDNA is relatively non-invasive, and patients do not need to tolerate a greater risk, causing patients to have a better willingness and compliance for ctDNA detection (20). Therefore, ctDNA is highly preferable for genetic testing in patients with acquired drug resistance over puncture biopsy. Hence, the NGS detection of ctDNA was found to be more convenient to dynamically monitor the real-time gene information of patients’ tumors, so as to achieve a more precise treatment (21).
Another advantage of NGS is that it can simultaneously detect multiple genes. In addition to point mutation and insertion/deletion, it can also detect copy number variation and chromosome rearrangement (22). Moreover, it is able to effectively use limited tissue samples to find out classical T790M and other non-T790M drug resistance mechanisms, which are particularly important for patients with advanced NSCLC. In this study, the mechanism of acquired resistance to first-line osimertinib, including MET gene amplification, was detected, which provided a therapeutic insight for patients with advanced NSCLC.
Radiotherapy is one of the main means of local treatment (23). With the increasing popularity of stereotactic radiotherapy, the accuracy of radiotherapy in treatment of lesions can be effectively improved, and the incidence of radiation damage can be reduced. According to the presented case, radiotherapy plays a significant role in the treatment of patients with locally advanced resectable NSCLC. After 7 months of treatment with Erlotinib, bone and brain metastasis occurred in September 2016. After whole brain radiotherapy, the patient’s tumor size decreased, while the chest lesions enlarged; chest radiotherapy was thus performed, in which the lung mass was reduced. These two occasions of radiotherapy use kept the disease under control to a certain extent and prolonged the duration of the application of erlotinib. Additionally, 3 months after the first use of osimertinib, the patient developed right cervical lymph node metastasis and left scapular metastasis. The effective duration for the use of osimertinib was prolonged for 4 months by radiotherapy of the neck and left shoulder joints and treatment with zoledronic acid. Therefore, it can be concluded that adjuvant radiotherapy plays an important role in disease control and can relieve the enlargement and metastasis of local tumors while also prolonging the action time of targeted drugs.
In conclusion, dynamic monitoring of tumor genomic profiles can detect the driving genes and drug resistance mechanisms and thus guide cancer treatment. In this study, the total survival time of NSCLC patients in stage IVA after radiotherapy and targeted therapy was found to be more than 3 years, indicating the significance of dynamic monitoring of gene mutations for cancer treatment.
Acknowledgments
The authors thank Shanghai Tongshu Biotechnology Co., Ltd. for technical support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159-67. [Crossref] [PubMed]
- Engelman JA, Jänne PA. Factors predicting response to EGFR tyrosine kinase inhibitors. Semin Respir Crit Care Med 2005;26:314-22. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Chen G, Feng J, Zhou C, et al. Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomised, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC). Ann Oncol 2013;24:1615-22. [Crossref] [PubMed]
- Pao W, Hutchinson KE. Chipping away at the lung cancer genome. Nature Medicine 2012;18:349-51. [Crossref] [PubMed]
- Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin 2015;25:185-97. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Ou SI, Agarwal N, Ali SM. High MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer 2016;98:59-61. [Crossref] [PubMed]
- Planchard D, Loriot Y, André F, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol 2015;26:2073-8. [Crossref] [PubMed]
- Ahn S, Hwang SH, Han J, et al. Transformation to Small Cell Lung Cancer of Pulmonary Adenocarcinoma: Clinicopathologic Analysis of Six Cases. J Pathol Transl Med 2016;50:258-63. [Crossref] [PubMed]
- Bearz A, De Carlo E, Doliana R, et al. Acquired BRAF V600E Mutation as Resistant Mechanism after Treatment with Third-Generation EGFR Tyrosine Kinase Inhibitor. J Thorac Oncol 2017;12:e181-2. [Crossref] [PubMed]
- Remon J, Morán T, Majem M, et al. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: a new era begins. Cancer Treat Rev 2014;40:93-101. [Crossref] [PubMed]
- Sacher AG, Jänne PA, Oxnard GR. Management of acquired resistance to epidermal growth factor receptor kinase inhibitors in patients with advanced non-small cell lung cancer. Cancer 2014;120:2289-98. [Crossref] [PubMed]
- Ortiz-Cuaran S, Scheffler M, Plenker D, et al. Heterogeneous Mechanisms of Primary and Acquired Resistance to Third-Generation EGFR Inhibitors. Clin Cancer Res 2016;22:4837-47. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]
- Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 2014;6:224ra24. [Crossref] [PubMed]
- Corcoran RB, Chabner BA. Application of Cell-free DNA Analysis to Cancer Treatment. N Engl J Med 2018;379:1754-65. [Crossref] [PubMed]
- Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet 2016;17:333-51. [Crossref] [PubMed]
- Le Péchoux C. Role of postoperative radiotherapy in resected non-small cell lung cancer: a reassessment based on new data. Oncologist 2011;16:672-81. [Crossref] [PubMed]