
Lung cancer in Spain: information from the Thoracic Tumors Registry (TTR study)
Introduction
Lung cancer remains a leading cause of cancer incidence and mortality worldwide among both men and women, with more than 2 million newly diagnosed cases and 1.8 million deaths in 2018, accounting for close to 1 in 5 (18.4%) cancer deaths (1). Lung cancer is broadly divided into two categories according to its histological characteristics: small cell lung cancer (SCLC, ~15% of all lung cancers) and non-small cell lung cancer (NSCLC, ~85%). The latter comprises several histological subtypes, mainly squamous cell carcinoma, adenocarcinoma and large-cell lung cancer; adenocarcinoma is the most common subtype of NSCLC (~40%) (2,3).
The most important risk factor for lung cancer is tobacco smoking, along with other environmental pollutants (4,5). Nevertheless, only approximately 10% of smokers develop lung cancer, and the disease also occurs in the absence of exposure to cigarette smoke (6); in this sense, several studies have identified a genetic susceptibility locus for lung cancer carcinogenesis and prognosis (7). Since these risk factors are highly preventable, mortality rates can be largely reduced through tobacco control and other population-based preventive strategies (8). Global numbers show a declining trend in the incidence and mortality rates in men, primarily due to decreased cigarette consumption. Among women, the tobacco epidemic is less advanced, and most countries are still observing a rising trend in incidence; only relatively few populations (e.g., the US and possibly the UK) are showing signs of decline among recent birth cohorts (1,9).
Information from European registries, including treatments for lung cancer and their efficacy, as well as data on tobacco consumption and mutational profiles of tumors, is somewhat scarce. Many countries collect data on a national level, with the majority using a national registry for all cancers, but few have a data collection program for lung cancer in addition to a cancer registry (10). However, this information is of particular interest, not only because of the need for deeper understanding of the characteristics of lung cancer, both in its presentation and during treatment, but also to evaluate certain aspects related to health care quality. In this sense, information from a country like Spain, with universal health coverage, can provide valuable data from a more realistic scenario, with no bias related to private health insurance or patient socioeconomic status.
According to recent data from the Spanish National Institute of Statistics (INE), lung cancer was responsible for the highest number of deaths among the Spanish population in 2017, and for the first time was the second cause of cancer mortality among Spanish women, especially due to smoking (11); the number of deaths in 2017 increased by 6.4% compared to the previous year, and doubled compared to 2003 (12).
Until recently, the coverage of the population by cancer registries in Spain was limited, with no official, unified database for lung cancer and other thoracic tumors. To obtain a better picture of the epidemiology of these diseases in Spain, in 2016 the Spanish Lung Cancer Group (GECP) created the first Thoracic Tumor Registry (TTR), an observational, prospective cohort multicenter study that included patients treated for lung cancer and other thoracic tumors. To our knowledge, this is the first registry of its kind in Europe. In this paper, we present the methodology of the registry and the results from 6,600 patients with NSCLC recruited in 56 Spanish hospitals until December 2018.
Methods
Study design
The TTR is an observational (patient registry), non-post-authorization, prospective cohort multicenter study. Enrolment started in August 2016 and is still ongoing (as of April 23, 2019, 10,145 patients from 58 centers had been included in the study, 8,653 of whom had been diagnosed with NSCLC). The study was conducted in accordance with the provisions of the Declaration of Helsinki. Protocol approval was obtained from the institutional review board at each study site. The registry was approved in 2016 by the Spanish Agency for Medicines and Medical Devices (AEMPS) and is registered on the ClinicalTrials.gov database (NCT02941458). Protocol approval was obtained from the institutional review board of Hospital Universitario Puerta de Hierro Majadahonda (No. PI 148/15).
Study sponsor
This registry was sponsored by the GECP, an independent, cooperative and multidisciplinary oncology group established in 1991, whose purpose is to promote the study and research of lung cancer and incorporate advances in the treatment of the disease into routine clinical practice. The GECP consists of more than 400 specialists from all over Spain associated with treatment and research in lung cancer, mostly medical oncologists. It brings together a network of more than 160 public and private hospitals distributed throughout the Spanish territory that conduct their research in a coordinated manner. This infrastructure was the basis for establishing the TTR registry, proposed by the steering committee.
Eligibility
Eligible patients included those with lung cancer or other types of thoracic tumors (NSCLC, SCLC, mesothelioma, thymic carcinoma, carcinoid cancer) undergoing active treatment or not treated (palliative care only), with no sex or age restrictions. Patients with other types of tumors and healthy volunteers were not admitted.
Information retrieval
Data was collected from patient medical records using an electronic data capture system (EDC), where each research team included the information on all patients with lung carcinoma attended by the healthcare personnel of their hospital. Sociodemographic, epidemiological, clinical, molecular/genetic and treatment outcome (e.g., response rate, current status, date of death) variables were recorded. The information collected was classified into different sections: (I) patient personal history, which included the performance status (PS), tobacco consumption (including environmental exposure to tobacco smoke), and comorbidities; (II) family history of cancer; (III) diagnosis, which included the histological type and detailed TNM classification of the tumor (including the location of metastases when appropriate); (IV) treatment, which included information on all treatments received, including dates and specific characteristics (surgery, chemotherapy, radiotherapy); (V) disease progression; and (VI) genetic information (alterations of driver genes).
Statistical analysis
Descriptive statistics were performed and quantitative data were summarized as mean, standard deviation (SD), median, interquartile range (IQR), minimum, and maximum. Qualitative variables were summarized as frequencies in the entire population and percentages. Characteristics of two groups (early and advanced) were compared using the χ2 test for categorical variables. The significance level was established at a value of α=0.05.
Results
Patient characteristics
A total of 6,600 patients diagnosed with NSCLC were enrolled between August 2016 and December 2018 in 56 hospitals of 12 Spanish autonomous regions. The median number of patients/month (patients/year) recruited in 2017 and 2018 was 162 [1,900] and 402 [4,423], respectively. Enrolled patients were diagnosed with NSCLC from before 2010 to 2018, with approximately 50% of them diagnosed in the last 3 years [2016–2018].
Baseline patient characteristics are shown in Table 1. There were significantly more males (73.4%) than females (26.6%). Median age at diagnosis was 64.0 years, with approximately 30% of patients being younger than 55 or older than 75 years. Regarding tobacco use, more than 85% of the patients diagnosed (n=5,650) were current smokers (n=2,611, 39.6%) or ex-smokers (n=3,039, 46.0%), and only 13.1% (n=866) had never smoked. The most frequent histological type was adenocarcinoma (n=4,208, 63.8%), followed by squamous cell carcinoma (n=1,826, 27.7%) and large cell carcinoma (n=202, 3.1%). More than 85% of patients presented a good PS [Eastern Cooperative Oncology Group (ECOG) PS of 0 or 1].
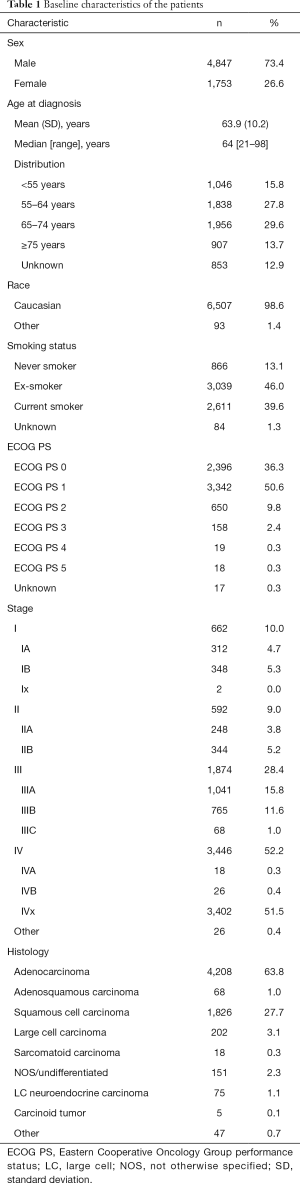
Full table
Patient characteristics according to disease stage
Median age at diagnosis was 64 years for patients with advanced disease and 65 for those with early-stage disease. As expected, most patients presented with advanced stage III (n=1,874, 28.4%) or IV (n=3,446, 52.2%) disease at diagnosis, independently of sex (63.1% and 70.6% in males and females, respectively). Interestingly, among those diagnosed with advanced disease, a progressive growth in the percentage of adenocarcinoma was observed over the years of diagnosis: 11% in ≤2012, 16% in 2013/2014, 28% in 2015/2016 and 32% in 2017/2018. There was a higher number of patients with no comorbidities at diagnosis among those with advanced disease than among those with early-stage disease (63.4% vs. 36.6%, P<0.001), although among patients who presented comorbidities, the prevalence was much higher, in general, in those with advanced disease. Significant differences (P<0.001, unless otherwise indicated) were observed between the two groups in the prevalence of cardiopathy, dyslipidemia (P=0.043), chronic obstructive pulmonary disease (COPD), hypercholesterolemia (P=0.026), hypertension, nephropathy (P=0.012), obesity (P=0.007), and vasculopathy (Table 2). Likewise, the prevalence of symptoms, including cough, pain, dyspnea, hemoptysis, weight loss, anorexia, and asthenia was much higher (P<0.001) among patients with advanced disease (Table S1).
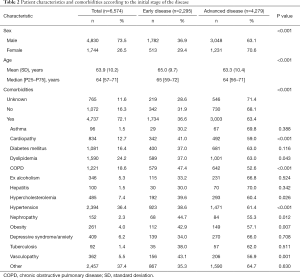
Full table
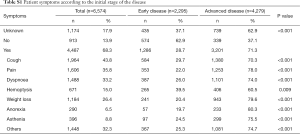
Full table
Although no association was found between the professional occupation of patients (recorded in only 20% of cases) and the disease stage at diagnosis (data not shown), differences were found according to previous exposure to potential carcinogenic compounds. Compared to the total population, a higher percentage of patients with advanced disease was observed among those who had been exposed to arsenic compounds (87.5% vs. 65.1%, P=0.020) and, to a lesser extent, acrylonitrile (75.0% vs. 65.1%, P=0.020); on the other hand, early-stage disease was more prevalent among patients exposed to asbestos (47.5% vs. 34.9%, P=0.020), radon/silica (47.4% vs. 34.9%, P=0.020) and, to a lesser extent, paint (38.1% vs. 34.9%, P=0.020) and diesel engine smoke (38.2% vs. 34.9%, P=0.020) (Table S2).
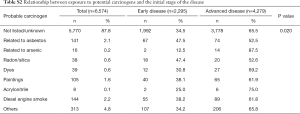
Full table
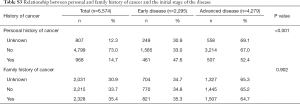
More patients with a previous history of cancer had early-stage disease at diagnosis than those with advanced disease (47.6% vs. 34.9%, P<0.001); no differences were found in the disease stage at diagnosis according to family history of prostate cancer (Table S3).
Full table
Smoking status
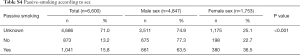
The majority of patients included in the study, 86.7%, were current smokers or ex-smokers (40.1% and 46.6%, respectively). Only 13.3% had never smoked (Table 3). In addition, almost 16% of patients were passive smokers (i.e., those who lived with active smokers in the last 20 years), with proportionally higher percentage among women (Table S4).

Full table
Full table
Smoking status according to sex
Comparing smoking status by sex, significant differences were observed between males and females in all groups, non-smokers, ex-smokers and current smokers (P<0.001). The percentage of non-smokers was much higher in females (37.7%) than in males (4.5%), while the percentage of ex-smokers was much higher in males (53.4%) than in females (27.9%), as was the percentage of current smokers (42.1% vs. 34.4%, respectively). These sex differences were maintained throughout all the years of diagnosis. In males, a rapidly growing trend in the number of patients who were active smokers was observed from 2015 to 2018, along with an opposite downward trend in the number of ex-smokers; the low percentage of males who never smoked remained stable throughout the period analyzed. In females, a rapid decline in the number of patients who never smoked was observed from 2011 onwards, along with a continuous rise in the number of current smokers; although much higher than in males, the percentage of ex-smokers remained more or less stable during the period analyzed (Table 3).
Age of onset of smoking
The age of onset of smoking was recorded in 2,707 patients (47.9% of total current smokers and ex-smokers), with the average being 18.2 years in the total population. There were significant differences (P<0.001) between males and females, with males being earlier to start smoking (mean age 17.9 years; 95% CI: 17.6–18.2) than females (mean age 19.2 years; 95% CI: 18.5–19.8) (Figure 1A). The differences in the average age of onset of smoking between sexes remained more or less stable throughout all the years of diagnosis (Figure 1B).

Smoking status according to disease stage
No association was observed between the smoking status, active or passive, and the initial stage of the disease. In all subgroups, the percentage of patients presenting with advanced disease was significantly higher (active smoking, P<0.001; passive smoking, P=0.003) than that of patients with early disease (Table 4).

Full table
Biomarker profiling of tumors
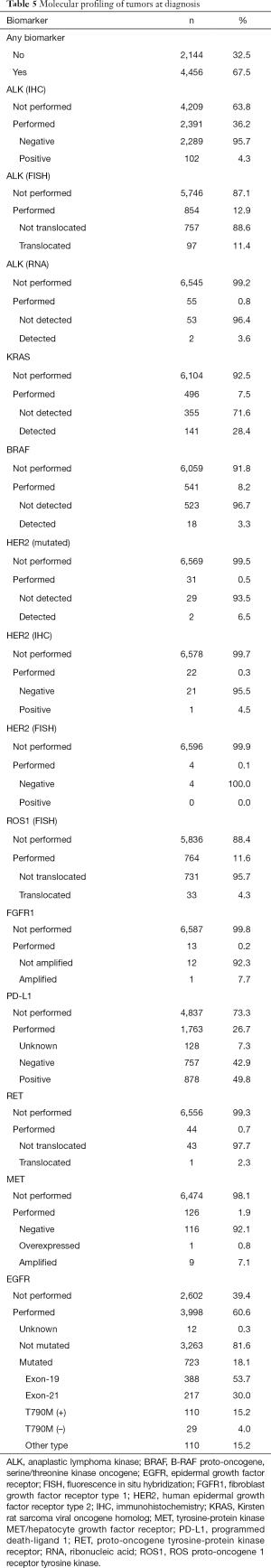
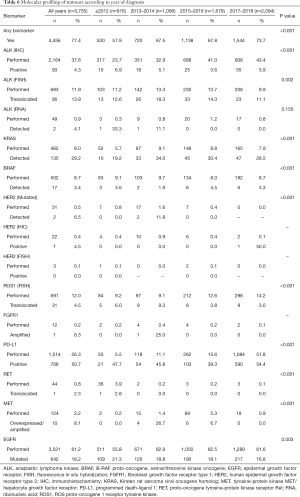
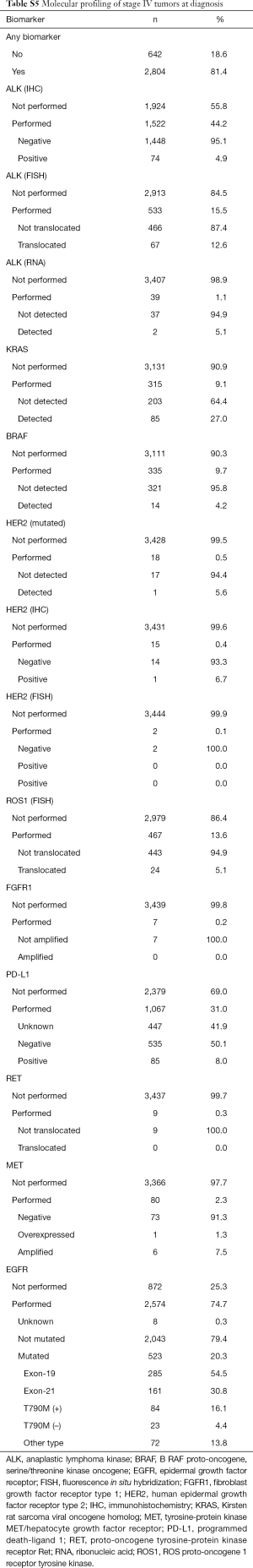
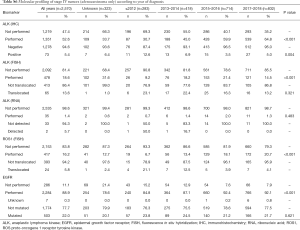
A total of 4,456 patients (67.5%) underwent some type of molecular testing for biomarker analysis, including epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), Kirsten rat sarcoma viral oncogene homolog (KRAS), B-RAF proto-oncogene, serine/threonine kinase oncogene (BRAF), human epidermal growth factor receptor type 2 (HER2), ROS proto-oncogene 1, receptor tyrosine kinase (ROS1), fibroblast growth factor receptor type 1 (FGFR1), programmed death-ligand 1 (PD-L1), proto-oncogene tyrosine-protein kinase receptor Ret (RET), and tyrosine-protein kinase MET/hepatocyte growth factor receptor (MET). EGFR mutational profiling was the most frequent test, performed in about 60% of the population. Mutations in EGFR were detected in 18.1% of patients, including in-frame deletions in exon 19 (53.7%), point mutations in exon 21 (30.0%), and T790M mutation in exon 20 of the kinase domain (15.2%), among other mutations (15.2%). KRAS mutations were detected in 28.4% of patients (Table 5). The total percentage of biomarker characterization increased from 57.9% before 2013 to 73.7% in 2017/2018. Particularly relevant in this period was the increase in the percentage of characterization of PD-L1 (from 5.5% to 51.8%) and ALK (from 35.2% to 54.1%) (Table 6). In general, the mutational profile of advanced, stage IV tumors (n=3,446, 52.2%) was rather similar to that of the total set of tumors, regardless of the disease stage (Table S5). There were also differences in the percentage of biomarker characterization in advanced tumors (adenocarcinoma) according to the year of diagnosis. A progressive increase from before 2013 to 2017/2018 was observed in the percentage of ALK characterization (immunohistochemistry, IHC), from 30.7% to 64.8% (P<0.001) and ROS1 (fluorescence in situ hybridization, FISH), from 6.7% to 20.7% (P<0.001). In addition, a much higher percentage of positive results for ALK rearrangements (IHC) was observed before 2013, compared to later years. The percentage of EGFR characterization, although high throughout all the years of follow-up, showed a progressive increase from 84.8% before 2013 to 92.1% in 2017/2018 (P<0.001) (Table S6).
Full table
Full table
Full table
Full table
Discussion
In our opinion, it is essential to have information on patients that allows the current situation of lung cancer in different European regions to be compared. Although the EUROCARE study attempts to monitor and identify the survival of cancer patients in Europe, it only covers about 1% of the population and has reported potentially important regional variations (13). At country level, several initiatives have been carried out in European countries. In England, for example, the National Lung Cancer Audit (NLCA) was established in 2004 to identify possible inequalities within the National Health Service and to highlight the potential for service improvements (14); in Denmark, the Danish Lung Cancer Registry (DLCR) was set up in 1991 to improve survival and clinical management of Danish lung cancer patients, as well as to produce a platform for lung cancer research (15); and in Sweden, the National Quality Registry for Lung Cancer was established in 2002 to provide information on diagnostic procedures, staging methods, tumor characteristics, planned treatment, study participation and follow-up (16). In addition, registry-based studies have been published in Norway (17), France (18), Germany (19) and the Netherlands (20). In Spain, however, there has been no national hospital clinical register to date.
The TTR study reflects the current epidemiology and treatment of lung cancer in Spain, based on a very large sample size. Among the main findings, we observed that, at the time of diagnosis, the median age was 64 years and 52% of patients presented with advanced stage IV disease. Despite the fact that the majority of cases corresponded to males (about 73%), females already account for around 1 in 4 cases of lung cancer (27%), following the upward global trend observed in recent years (21). Data published in 2017 showed that, among all countries with an increasing incidence among women, Spain had the second highest average annual percentage change (8.2, 95% CI: 6.6–9.9; P<0.001) in the last 10 years (22).
Tobacco use was a risk factor that was present in 86% of our patients, with 46% being active smokers (42% among males and 34% among females) at the time of diagnosis, confirming that tobacco smoke is the main cause of this type of tumor (4,21). Comparing tobacco use by sex, striking differences were observed, with a higher percentage of male ex-smokers (nearly twice that of females) and a very much higher percentage (more than 8-fold) of female never-smokers. The percentage of active female smokers, although slightly less, was dangerously close to that of males. These data are somewhat worrying, since, although not without controversy, several studies have argued that women are more vulnerable to tobacco carcinogens (23,24) and are more predisposed than men to molecular aberrations resulting from the carcinogenic effects of tobacco smoke (25). Furthermore, female smokers are more likely than male smokers to develop adenocarcinoma of the lung, and those women who have never smoked are more likely to develop lung cancer than men (26). Considering the entire study population, the average age of onset of smoking was 18.2 years, with males starting to smoke significantly earlier; however, among women, a much higher percentage of later onset of smoking—from 16 to 19 years old—was observed, supporting the concern about the dangerous growing tendency of women to start smoking. Additionally, we must take into account that passive smoking causes many of the same diseases as direct smoking, and is a risk factor for lung cancer as well as other types of cancer (27). In this study, and in line with this evidence, a lower percentage of patients with early-stage disease at diagnosis were non-passive smokers. It should be noted that a higher than expected percentage of passive smokers was found among females. In our opinion, and in view of these results, the social and political pressure to reduce tobacco consumption among the population should be reinforced, particularly among youth.
Our data highlight the change of histological subtype presentation already suggested in EUROCARE studies (13). Although these studies have limitations derived from the high number of patients with non-specific histological subtypes and lack of diagnostic uniformity between different countries, it seems clear that there is an increasing frequency of adenocarcinoma (26,28,29), which was also the subtype most frequently observed in the TTR study. Since the 1970s in the US, adenocarcinoma as a percentage of all lung carcinomas has nearly doubled in men and increased from ~25% to ~33% in women, among whom adenocarcinoma has long been the most commonly diagnosed histological type (30). Although it has not been fully demonstrated, the decrease in tars and increase in nitrosamines in filtered cigarettes has been suggested as the cause of the recent change of dominant cell type from squamous cell to adenocarcinoma (31).
Regarding the presentation of the disease at diagnosis, our data were comparable to those from registries from the US, Canada and Australia, as well as European countries, such as the UK, Denmark, Norway and Sweden. However, it is worth noting the earlier diagnosis in our country, at mean age 64 vs. ~70 years in most of these countries, which is probably due to more complete, globalized healthcare coverage (32-35).
The molecular characterization of lung cancer has considerably changed the classification and treatment of these tumors, becoming an essential component of pathologic diagnosis and oncologic therapy decisions (36). In this study, just over two thirds of patients (67.5%) underwent molecular testing, reaching the significant percentage of 81.4% in patients with stage IV disease. It is worth highlighting not only the high percentage of EGFR mutation testing, but also the progressive and rapid increase in some biomarker profiling, as was the case of PD-L1 expression and determination of ALK rearrangements.
Mutations in EGFR were detected in almost one fifth of patients (18%), a percentage that is comparable with that previously reported for Caucasian patients with lung adenocarcinoma (37,38). EGFR is the most important driver gene in NSCLC (37), and tumors bearing EGFR mutations can be treated with first-line targeted therapies, such as the tyrosine kinase inhibitors (TKIs) gefitinib, erlotinib, afatinib, and osimertinib, leading to higher response rates (55–78%) than with standard chemotherapy (39). In about 15% of our patients, the so-called gatekeeper T790M mutation in exon 20 was detected, which is considered the most common resistance mechanism to TKIs (40).
Mutations in KRAS were detected in 28% of patients, a population that, according to published data, has a poor prognosis. The percentage of KRAS mutations in our population is also comparable with that reported for Caucasian patients with lung adenocarcinoma, estimated at around 30% (41). The KRAS pathway links the EGFR pathway to cell proliferation and survival, and KRAS mutations, which are associated with former or current smokers (41), have been shown to mediate resistance to TKIs, being a negative predictive factor in advanced NSCLC (42). ALK translocations were detected in 11% of patients included in the study. This percentage is somewhat higher than that previously published for NSCLC, ranging from 3% to 7.0% (43,44). The ALK fusion defines a distinct subpopulation of patients with lung adenocarcinoma who are highly responsive (57–74%) to ALK inhibitors such as crizotinib (45). It has been shown that EGFR, KRAS, and ALK molecular alterations are mutually exclusive events; nevertheless, they have been described in up to 2.7% of lung adenocarcinoma cases with concurrent molecular alterations (36). PD-L1 expression, which has been identified—although not without controversy—as a potential predictor of response to anti-PD-1 (e.g., pembrolizumab) and anti-PD-L1 (e.g., durvalumab) monoclonal antibody therapy and also as a prognostic biomarker, was detected in nearly 50% of patients in our cohort. A real-world study showed that, among patients with metastatic or recurrent NSCLC diagnosis eligible for the study, only 48% had one or more tests for PD-L1 determination, with 18% tested in 2015 and 71% in 2017 (46). These findings are important, since there are effective first-line therapies to treat patients with NSCLC who overexpress PD-L1 (47). In the clinical setting, correctly identifying these patients is imperative.
Other biomarkers analyzed showed percentages of positivity roughly similar to those previously published in adenocarcinoma, although some variability was expected due to the low number of patients evaluated: ROS1 rearrangements (observed vs. literature), 4.3% vs. 1–2%; MET amplification, 7.1% vs. 4–5%; RET fusions, 2.3% vs. 1–2%; BRAF mutations, 3.3% vs. 1–3%; HER2 mutations, 6.5% vs. 1.6–4%; and FGFR1 amplification, 7.7% vs. 3% (36,48).
Interestingly, the frequency of all these molecular alterations was similar when analyzed in the subgroup of patients with advanced stage IV disease. In this regard, a previous study by Pi et al. reported that early-stage and advanced-stage lung adenocarcinoma exhibited the same EGFR mutation frequencies and types (49).
A possible limitation of the study could be the potential bias due to the data sources, perhaps with greater representation of patients recruited in large hospitals. However, the study has many advantages, such as the large number of patients included to date and the considerable number of sites involved, of all sizes and from virtually the entire national territory; also important is the fact that Spain has a National Health System with universal coverage and, thus, all patients follow the same diagnostic work-up and have the same treatment opportunities, regardless of where they live or their income. A further advantage is that all patients have been recruited in a short-time period, allowing the comparison of treatments.
Conclusions
We believe that the TTR study accurately describes the clinical reality of lung cancer in Spain, including useful information with respect to demographic, clinical and molecular aspects that can be used to drive improvements in health care. In this sense, this type of studies should be extended to other European countries (50). Tobacco smoking, along with other environmental pollutants, remains the most important risk factor for lung cancer; social and political pressure to reduce tobacco consumption among the population should be reinforced, particularly among youth.
Acknowledgments
The authors received medical writing support in the preparation of this manuscript from Luis F. García-Fernández, PhD (Medical Statistics Consulting, S.L., Valencia, Spain).
Funding: This work was supported by Grupo Español de Cáncer de Pulmón (GECP), Novartis, Lilly and Merck Sharp & Dohme (MSD). The funders had no involvement in writing the article or the decision to submit the article for publication. The authors received no compensation for writing the manuscript.
Footnote
Conflicts of Interest: Dr. M Provencio has received personal fees from Bristol-Myers Squibb, Merck Sharp & Dohme (MSD), Roche, Novartis, and Takeda and research grants from Roche and Bristol-Myers Squibb. Dr. R López-Castro has received personal fees from Roche, Boehringer Ingelheim, AstraZeneca, Bristol Myers Squibb and Merck Serono and non-financial support from Roche and Bristol Myers Squibb. Dr. J Bosch-Barrera has received personal fees from Bristol-Myers Squibb, Boheringer Ingelheim, MSD, and Roche and research grants from Pfizer, Boheringer-Ingelheim, and Roche. Dr. M Domine has received personal fees from Abbvie, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, MSD, Pfizer, and Roche. The other authors no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the provisions of the Declaration of Helsinki. Protocol approval was obtained from the institutional review board at each study site. The registry was approved in 2016 by the Spanish Agency for Medicines and Medical Devices (AEMPS) and is registered on the ClinicalTrials.gov database (NCT02941458). Protocol approval was obtained from the institutional review board of Hospital Universitario Puerta de Hierro Majadahonda (No. PI 148/15).
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [Crossref] [PubMed]
- Li T, Kung HJ, Mack PC, et al. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49. [Crossref] [PubMed]
- Hecht SS, Szabo E. Fifty years of tobacco carcinogenesis research: from mechanisms to early detection and prevention of lung cancer. Cancer Prev Res (Phila) 2014;7:1-8. [Crossref] [PubMed]
- Loomis D, Grosse Y, Lauby-Secretan B, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol 2013;14:1262-3. [Crossref] [PubMed]
- Spinola M, Meyer P, Kammerer S, et al. Association of the PDCD5 locus with lung cancer risk and prognosis in smokers. J Clin Oncol 2006;24:1672-8. [Crossref] [PubMed]
- Guo NL, Tosun K, Horn K. Impact and interactions between smoking and traditional prognostic factors in lung cancer progression. Lung Cancer 2009;66:386-92. [Crossref] [PubMed]
- WHO Framework Convention on Tobacco Control Overview. Available online: http://www.who.int/fctc/text_download/en/index.html
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-87. [Crossref] [PubMed]
- Rich A, Baldwin D, Alfageme I, et al. Achieving Thoracic Oncology data collection in Europe: a precursor study in 35 Countries. BMC Cancer 2018;18:1144. [Crossref] [PubMed]
- Instituto Nacional de Estadística (INE). Defunciones según la Causa de Muerte - Año 2017. Available online: https://www.ine.es/prensa/edcm_2017.pdf
- Instituto Nacional de Estadística (INE). Defunciones según la Causa de Muerte - Año 2003. Available online: https://www.ine.es/prensa/np393.pdf
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol 2014;15:23-34. [Crossref] [PubMed]
- Rich AL, Tata LJ, Free CM, et al. Inequalities in outcomes for non-small cell lung cancer: the influence of clinical characteristics and features of the local lung cancer service. Thorax 2011;66:1078-84. [Crossref] [PubMed]
- Jakobsen E, Rasmussen TR. The Danish Lung Cancer Registry. Clin Epidemiol 2016;8:537-41. [Crossref] [PubMed]
- Nationella Kvalitetsregister. National Quality Registry for Lung Cancer. Available online: http://kvalitetsregister.se/englishpages/findaregistry/registerarkivenglish/nationalqualityregistryforlungcancer.2280.html
- Nilssen Y, Strand TE, Fjellbirkeland L, et al. Lung cancer survival in Norway, 1997-2011: from nihilism to optimism. Eur Respir J 2016;47:275-87. [Crossref] [PubMed]
- Chouaïd C, Debieuvre D, Durand-Zaleski I, et al. Survival inequalities in patients with lung cancer in France: A nationwide cohort study (the TERRITOIRE Study). PLoS One 2017;12:e0182798. [Crossref] [PubMed]
- Eberle A, Jansen L, Castro F, et al. Lung cancer survival in Germany: A population-based analysis of 132,612 lung cancer patients. Lung Cancer 2015;90:528-33. [Crossref] [PubMed]
- van der Drift MA, Karim-Kos HE, Siesling S, et al. Progress in standard of care therapy and modest survival benefits in the treatment of non-small cell lung cancer patients in the Netherlands in the last 20 years. J Thorac Oncol 2012;7:291-8. [Crossref] [PubMed]
- Martín-Sánchez JC, Lunet N, Gonzalez-Marron A, et al. Projections in Breast and Lung Cancer Mortality among Women: A Bayesian Analysis of 52 Countries Worldwide. Cancer Res 2018;78:4436-42. [Crossref] [PubMed]
- Wong MCS, Lao XQ, Ho KF, et al. Incidence and mortality of lung cancer: global trends and association with socioeconomic status. Sci Rep 2017;7:14300. [Crossref] [PubMed]
- International Early Lung Cancer Action Program Investigators, Henschke CI, Yip R, et al. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA 2006;296:180-4. [Crossref] [PubMed]
- Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 1996;88:183-92. [Crossref] [PubMed]
- Kure EH, Ryberg D, Hewer A, et al. p53 mutations in lung tumours: relationship to gender and lung DNA adduct levels. Carcinogenesis 1996;17:2201-5. [Crossref] [PubMed]
- Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol 2013;30:93-8. [Crossref] [PubMed]
- Cao S, Yang C, Gan Y, et al. The Health Effects of Passive Smoking: An Overview of Systematic Reviews Based on Observational Epidemiological Evidence. PLoS One 2015;10:e0139907. [Crossref] [PubMed]
- Hatcher J, Dover DC. Trends in histopathology of lung cancer in Alberta. Can J Public Health 2003;94:292-6. [PubMed]
- Travis WD, Lubin J, Ries L, et al. United States lung carcinoma incidence trends: declining for most histologic types among males, increasing among females. Cancer 1996;77:2464-70. [Crossref] [PubMed]
- Devesa SS, Shaw GL, Blot WJ. Changing patterns of lung cancer incidence by histological type. Cancer Epidemiol Biomarkers Prev 1991;1:29-34. [PubMed]
- Stellman SD, Muscat JE, Thompson S, et al. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer 1997;80:382-8. [Crossref] [PubMed]
- Bryan S, Masoud H, Weir HK, et al. Cancer in Canada: Stage at diagnosis. Health Rep 2018;29:21-5. [PubMed]
- National Cancer Institute (NCI). Surveillance, Epidemiology, and End Results Program (SEER). Available online: https://seer.cancer.gov/
- Thomas A, Chen Y, Yu T, et al. Trends and Characteristics of Young Non-Small Cell Lung Cancer Patients in the United States. Front Oncol 2015;5:113. [Crossref] [PubMed]
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax 2013;68:551-64. [Crossref] [PubMed]
- Villalobos P, Wistuba II. Lung Cancer Biomarkers. Hematol Oncol Clin North Am 2017;31:13-29. [Crossref] [PubMed]
- Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306-11. [Crossref] [PubMed]
- Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009;361:958-67. [Crossref] [PubMed]
- Hirsh V. Turning EGFR mutation-positive non-small-cell lung cancer into a chronic disease: optimal sequential therapy with EGFR tyrosine kinase inhibitors. Ther Adv Med Oncol 2018;10:1758834017753338. [Crossref] [PubMed]
- Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med 2005;2:e73. [Crossref] [PubMed]
- Suda K, Tomizawa K, Mitsudomi T. Biological and clinical significance of KRAS mutations in lung cancer: an oncogenic driver that contrasts with EGFR mutation. Cancer Metastasis Rev 2010;29:49-60. [Crossref] [PubMed]
- Garassino MC, Borgonovo K, Rossi A, et al. Biological and clinical features in predicting efficacy of epidermal growth factor receptor tyrosine kinase inhibitors: a systematic review and meta-analysis. Anticancer Res 2009;29:2691-701. [PubMed]
- Sholl LM. Biomarkers in lung adenocarcinoma: a decade of progress. Arch Pathol Lab Med 2015;139:469-80. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Velcheti V, Patwardhan PD, Liu FX, et al. Real-world PD-L1 testing and distribution of PD-L1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the United States. PLoS One 2018;13:e0206370. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res 2015;4:36-54. [PubMed]
- Pi C, Xu CR, Zhang MF, et al. EGFR mutations in early-stage and advanced-stage lung adenocarcinoma: Analysis based on large-scale data from China. Thorac Cancer 2018;9:814-9. [Crossref] [PubMed]
- Zanetti R, Sacchetto L, Coebergh JW, et al. To accelerate cancer prevention in Europe: Challenges for cancer registries. Eur J Cancer 2018;104:151-9. [Crossref] [PubMed]


