Adjuvant chemotherapy in patients with completely resected non-small cell lung cancer
Introduction
Approximately 25-30% of patients with non-small cell lung cancer (NSCLC) present with localized disease at the time of diagnosis and undergo surgery with curative intent. Despite complete tumor resection, however, many patients will develop systemic relapses with or without local relapses and will eventually die from their disease. Thus adjuvant chemotherapy was studied as a strategy to increase survival of patients with completely resected NSCLC. A meta-analysis of early trials revealed a statistically non-significant survival gain of 5% at 5 years by adjuvant cisplatin-based chemotherapy (1). These results then led to re-evaluation of adjuvant chemotherapy with modern platinum-based regimens within randomized phase III trials on large patient populations (2-8). Three trials and the meta-analysis of cisplatin-based trials then proved the survival benefit for cisplatin-based chemotherapy (2-5,9). These results then established adjuvant chemotherapy as standard treatment for patients with completely resected NSCLC. The present paper summarizes the pivotal trials, describes the current status of adjuvant chemotherapy, and also outlines future strategies to improve outcome of adjuvant therapy in patients with completely resected NSCLC.
Phase III trials
The meta-analysis of early adjuvant chemotherapy trials suggested a survival benefit for adjuvant chemotherapy and led to re-evaluation of adjuvant cisplatin-based chemotherapy with better chemotherapy regimens and/or better supportive care within large phase III trials in patients with completely resected NSCLC (2-8). The results of these trials are summarized in Table 1.
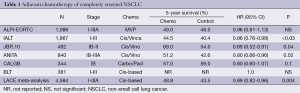
Full table
International adjuvant lung cancer trial (IALT)
IALT was the first trial that demonstrated a statistically significant improvement in overall survival by adjuvant cisplatin-based chemotherapy (2). The trial enrolled 1,867 patients and, therefore, was the largest adjuvant chemotherapy trial. The patients had the following baseline characteristics: median age 59; 80% males; WHO performance status 0-1 and 2 in 93% and 7%; 36.5% stage I, 24.2% stage II, 39.3% stage III; 47% squamous cell carcinomas, 40% adenocarcinomas, 13% large cell carcinomas and others; 64% lobectomy, 35% pneumonectomy, 2 in 74% of the patients. Cisplatin was combined with either etoposide (56%), vinorelbine (27%), vinblastine (11%) or vindesine (6%).
Adjuvant chemotherapy increased overall survival of the patients. The hazard ratio was 0.86 (95% CI, 0.76-0.98; P3). The results of IALT were consistent with those of the meta-analysis of early trials and led to the clinical implementation of adjuvant chemotherapy in patients with completely resected NSCLC.
JBR.10 trial
The JBR.10 trial (4) enrolled 482 patients with the following baseline characteristics: median age 61; 65% male; 53% adenocarcinomas; 45% stage IB, 55% stage II. Patients of the chemotherapy arm were planned to receive cisplatin (50 mg/m2 on days 1 and 8 every 4 weeks for four cycles) plus vinorelbine (25 mg/m2 weekly for 16 weeks). Fifty-eight percent of the patients received three or more cycles of cisplatin. Seventy-seven percent of patients required at least one dose reduction or omission.
Adjuvant chemotherapy increased overall survival of the patients. The hazard ratio was 0.69 (95% CI, 0.52-0.91). Five-year survival rates were 69% and 54% in the chemotherapy arm and control arm, respectively. Relapse-free survival was also increased. Side effects of chemotherapy included neutropenia (88% of the patients), febrile neutropenia (7%), fatigue (81%), nausea (80%), anorexia (55%), vomiting (48%), neuropathy (48%) and constipation (47%). Chemotherapy-associated mortality was 0.8%.
Patients who had undergone pneumonectomy were more likely to discontinue therapy due to toxicity (10). Elderly patients did also benefit at acceptable toxicity (11). Adjuvant chemotherapy was also considered to be cost effective (12).
ANITA trial
The ANITA trial (5) enrolled 840 patients with the following baseline characteristics: median age 59, 86% male, 35% stage IB, 30% stage II, 35% stage III; 58% lobectomy, 37% pneumonectomy. Patients of the chemotherapy arm were planned to receive cisplatin 100 mg/m2 on days 1, 29, 57 and 85 plus vinorelbine 30 mg/m2 weekly for a maximum of 16 doses. Fifty percent of the patients completed the planned four cycles. The dose intensities were 22 mg/m2 per week for cisplatin and 18 mg/m2 per week for vinorelbine.
Adjuvant chemotherapy improved survival of the patients. The hazard ratio was 0.80 (95% CI, 0.66-0.96). Five-year survival rates were 51% and 42.6%, and 7-year survival rates were 45.2% and 36.8% in the chemotherapy arm and control arm, respectively. Side effects of chemotherapy included neutropenia (92% of the patients), febrile neutropenia (9%) and nausea/vomiting (27% grade 3-4). Chemotherapy-associated mortality was 2%. This higher percentage compared to other trials can be explained by the higher drug doses used in the ANITA trial. Therefore, the authors suggested slightly lower drug doses for clinical practice than those of the ANITA trial.
Other adjuvant chemotherapy trials
The ALPI-EORTC failed to demonstrate a survival benefit for adjuvant chemotherapy with cisplatin, mitomycin C and vindesine (6). The toxicity of the in the meantime outdated chemotherapy protocol might have contributed to the failure of the trial. The Big Lung Trial had insufficient statistical power to prove or exclude the benefit of adjuvant chemotherapy (7).
The CALGB study enrolled 344 patients with completely resected stage IB NSCLC (8). Patients of the chemotherapy arm received paclitaxel plus carboplatin. Chemotherapy did not significantly increase overall survival. The hazard ratio was 0.8 (95% CI, 0.6-1.07). The 5-year survival rates were 59% and 57% in the chemotherapy arm and control arm, respectively. A subgroup analysis revealed that patients with tumors larger than 4 cm may derive a survival benefit from adjuvant chemotherapy. Chemotherapy did not result in treatment-related deaths.
A benefit of adjuvant therapy with uracil-tegafur has been reported for Japanese patients with stage IB NSCLC (13).
Meta-analyses
The lung adjuvant cisplatin evaluation (LACE) meta-analysis (9) included a total of 4,584 patients from the following five trials: ALPI-EORTC, IALT, JBR.10, ANITA and the Big Lung Trial. The analysis demonstrated a survival benefit for adjuvant cisplatin-based chemotherapy. The hazard ratio was 0.89 (95% CI, 0.82-0.96; P=0.004). The 5-year survival rate was increased by 5.3%. Disease-free survival was also increased by adjuvant chemotherapy with a hazard ratio of 0.8 (95% CI, 0.78-0.9; P
When only patients who had been treated with cisplatin plus vinorelbine were analyzed (LACE-vinorelbine cohort), the hazard ratio was 0.8 (95% CI, 0.70-0.91) and the increase in the 5-year survival rate was 8.9% (14).
Impact on clinical practice
Adjuvant chemotherapy increased survival of patients with completely resected NSCLC in three phase III trials (2-5). Within these trials, adjuvant chemotherapy resulted in an absolute increase in the 5-year survival rates ranging from 4% to 15% and in hazard ratios for death ranging from 0.69 to 0.86 (2-5). Cisplatin plus vinorelbine was the most widely used chemotherapy protocol. All patients of the JBR.10 trial and the ANITA trial and 27% of IALT patients were treated with this regimen in the chemotherapy arm. The chemotherapy-associated mortality was 1-2%.
The survival benefit was then confirmed by the LACE meta-analysis (9). Based on these phase III trials and the meta-analysis, adjuvant chemotherapy after complete resection of NSCLC stages II and III has been established as standard of care in patients with good performance status, rapid postoperative recovery, adequate organ function and informed consent. Patients should receive four cycles of a cisplatin-based doublet, preferably cisplatin plus vinorelbine. The chemotherapy should start 4-8 weeks after surgery.
Adjuvant chemotherapy can also be considered for selected patients with stage IB (sixth TNM classification) (15). Factors supporting adjuvant chemotherapy in these patients are younger age, good performance status, patient preference, large tumors, visceral pleural invasion and inadequate staging.
Perspectives of adjuvant therapy
Three major strategies are investigated for improving outcome of adjuvant therapy in patients with completely resected NSCLC. The first strategy focuses on characterization of predictive biomarkers and customized chemotherapy. The second strategy assesses the integration of targeted therapies. The third strategy evaluates tumor vaccines.
Predictive biomarkers and customized chemotherapy
Translational research has focussed on the characterization of biomarkers for the selection of patients for adjuvant chemotherapy. Predictive biomarkers for characterizing patients benefiting from adjuvant therapy are of greater interest than prognostic biomarkers. The focus has been on molecular tumor features which are involved in either tumor growth or mechanisms of action of anticancer drugs. In particular, DNA repair enzymes, drug transporters, apoptosis parameters and cell cycle regulators have been studied as potential biomarkers.
The IALT biologic program (IALT-Bio) has attempted to characterize predictive biomarkers from IALT. Many molecular tumor factors have been studied for their potential role as biomarkers. Patients with low ERCC1 expression in their tumors were shown to benefit from adjuvant chemotherapy, whereas those with high ERCC1 expression did not (16). Similarly, patients without p27 expression in their tumors did benefit from adjuvant chemotherapy (17). In contrast to ERCC1 and p27, multidrug resistance protein expression was without predictive value (18). However, neither ERCC1 nor p27 could be validated as predicted biomarkers in the LACE-Bio project. The lack of validation of ERCC1 as predictive biomarker might be due to changes in the anti-ERCC antibody over time (19).
Translational research of the JBR.10 trial also focussed on the characterization of biomarkers (20-22). High class III beta tubulin expression was associated with shorter relapse-free and overall survival in patients treated with surgery alone but not in patients treated with surgery plus adjuvant chemotherapy (20). The survival benefit of adjuvant chemotherapy was greater in patients with high compared to those with low tubulin expression in their tumors. However, the interaction between tubulin expression and chemotherapy treatment did not reach statistical significance (20). Patients with overexpression of p53 protein in their tumors had shorter survival but greater benefit from adjuvant chemotherapy than patients without overexpression of p53 (21). Lower haemoglobin levels at baseline were associated with a trend for shorter overall survival (22).
Customized chemotherapy based on molecular tumor characteristics is currently evaluated within phase III trials. The Spanish lung cancer group investigates adjuvant chemotherapy customized according to BRCA1 mRNA levels. The ITACA trial studies customized chemotherapy based on ERCC1 and thymidylate synthase levels. Until its clinical usefulness will have been proven in these or other trials, customized chemotherapy will remain experimental.
Integration of targeted therapies
The second strategy to improve outcome of adjuvant chemotherapy studies the integration of targeted therapies. EGFR-directed therapies and angiogenesis inhibitors are of primary interest because of their efficacy in patients with advanced NSCLC.
EGFR-directed TKIs as single agents have shown efficacy in patients previously treated with chemotherapy, in the maintenance setting and in patients with EGFR-activating mutations independent of treatment line (23). A multi-center, randomized, double-blind, placebo-controlled, phase III trial (RADIANT) compared single-agent erlotinib with placebo following complete tumor resection with or without adjuvant chemotherapy in patients with stage IB-IIIA NSCLC who have EGFR-positive tumors based on FISH and/or immunohistochemistry (24). However, disease-free survival and overall survival were not different between the erlotinib and the placebo arm. Among patients with EGFR mutation-positive NSCLC, disease-free survival was better in the erlotinib arm compared to the placebo arm but this difference might have been caused by imbalances in prognostic factors between the two arms. Similarly, adjuvant therapy with gefitinib failed to improve disease-free survival or overall survival in another trial (25). Trials with adjuvant gefitinib in patients with EGFR mutation-positive tumors are currently ongoing. Cetuximab has shown efficacy in patients with advanced NSCLC, particularly in those with high EGFR levels in their tumors (26,27). Despite these findings, no anti-EGFR monoclonal antibody is currently evaluated in the adjuvant setting.
Bevacizumab added to adjuvant chemotherapy is currently evaluated in the North American Intergroup Adjuvant Chemotherapy Trial ECOG 1505 in patients with early-stage NSCLC. In this trial, patients with stage IB (>4 cm), II or IIIA NSCLC will be randomized to receive adjuvant cisplatin-based chemotherapy either alone or in combination with bevacizumab.
Vaccines
The third strategy to improve outcome of completely resected NSCLC focuses on immunological approaches. MAGE-A3 is an interesting target for such an approach because it can be detected in about 35% of early-stage NSCLC but not in normal cells. Results from adjuvant trials with a MAGE-A3 vaccine have recently been reported (28-30). A randomized, placebo-controlled phase II trial indicated that vaccination with MAGE-A3 vaccine in MAGE-A3 positive patients with stage IB or II NSCLC reduces the relative risk of recurrence by 27% (28). In addition, a gene signature allowed characterizing those patients who will derive a benefit from the vaccine (29). According to a press release (30), however, the phase III trial (MAGRIT) failed to demonstrate an improvement in disease-free survival for the vaccine compared to placebo.
Conclusions
Adjuvant chemotherapy, preferentially with four cycles of cisplatin plus vinorelbine, has been established as a standard for patients with completely resected NSCLC. Clinical trials currently study bevacizumab added to adjuvant chemotherapy, gefitinib in patients with EGFR mutation-positive tumors, and customized chemotherapy based on molecular tumor features.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909. [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [PubMed]
- Arriagada R, Dunant A, Pignon JP, et al. Long-term results of the international adjuvant lung cancer trial evaluating adjuvant Cisplatin-based chemotherapy in resected lung cancer. J Clin Oncol 2010;28:35-42. [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [PubMed]
- Scagliotti GV, Fossati R, Torri V, et al. Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J Natl Cancer Inst 2003;95:1453-61. [PubMed]
- Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173-82. [PubMed]
- Strauss GM, Herndon JE 2nd, Maddaus MA, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol 2008;26:5043-51. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Alam N, Shepherd FA, Winton T, et al. Compliance with post-operative adjuvant chemotherapy in non-small cell lung cancer. An analysis of National Cancer Institute of Canada and intergroup trial JBR.10 and a review of the literature. Lung Cancer 2005;47:385-94. [PubMed]
- Pepe C, Hasan B, Winton TL, et al. Adjuvant vinorelbine and cisplatin in elderly patients: National Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol 2007;25:1553-61. [PubMed]
- Ng R, Hasan B, Mittmann N, et al. Economic analysis of NCIC CTG JBR.10: a randomized trial of adjuvant vinorelbine plus cisplatin compared with observation in early stage non-small-cell lung cancer--a report of the Working Group on Economic Analysis, and the Lung Disease Site Group, National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:2256-61. [PubMed]
- Kato H, Ichinose Y, Ohta M, et al. A randomized trial of adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. N Engl J Med 2004;350:1713-21. [PubMed]
- Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the Lung Adjuvant Cisplatin Evaluation. J Thorac Oncol 2010;5:220-8. [PubMed]
- Horn L, Sandler AB, Putnam JB Jr, et al. The rationale for adjuvant chemotherapy in stage I non-small cell lung cancer. J Thorac Oncol 2007;2:377-83. [PubMed]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Filipits M, Pirker R, Dunant A, et al. Cell cycle regulators and outcome of adjuvant cisplatin-based chemotherapy in completely resected non-small-cell lung cancer: the International Adjuvant Lung Cancer Trial Biologic Program. J Clin Oncol 2007;25:2735-40. [PubMed]
- Filipits M, Haddad V, Schmid K, et al. Multidrug resistance proteins do not predict benefit of adjuvant chemotherapy in patients with completely resected non-small cell lung cancee: International Adjuvant Lung Cancer Trial Biologic Program. Clin Cancer Res 2007;13:3892-8. [PubMed]
- Friboulet L, Olaussen KA, Pignon JP, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med 2013;368:1101-10. [PubMed]
- Sève P, Lai R, Ding K, et al. Class III beta-tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non-small cell lung cancer: analysis of NCIC JBR.10. Clin Cancer Res 2007;13:994-9. [PubMed]
- Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol 2007;25:5240-7. [PubMed]
- Gauthier I, Ding K, Winton T, et al. Impact of hemoglobin levels on outcomes of adjuvant chemotherapy in resected non-small cell lung cancer: the JBR.10 trial experience. Lung Cancer 2007;55:357-63. [PubMed]
- Pirker R, Minar W, Filipits M. Integrating epidermal growth factor receptor-targeted therapies into platinum-based chemotherapy regimens for newly diagnosed non-small-cell lung cancer. Clin Lung Cancer 2008;9 Suppl 3:S109-15. [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. A randomized, double-blind phase 3 trial of adjuvant erlotinib (E) versus placebo (P) following complete tumor resection with or without adjuvant chemotherapy in patients (pts) with stage IB-IIIA EGFR positive (IHC/FISH) non-small cell lung cancer (NSCLC): RADIANT results. J Clin Oncol 2014;32:abstr 7501.
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol 2012;13:33-42. [PubMed]
- Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013;31:2396-403. [PubMed]
- Ulloa-Montoya F, Louahed J, Dizier B, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 2013;31:2388-95. [PubMed]
- Available online: http://www.gsk.com/media/press-releases/2014/update-on-phase-III-clinical-trial-of-investigational-MAGE-A3-antigen-specific-cancer-immunotherapeutic-in-non-small-cell-lung-cancer.html


