Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients with advanced non-small cell lung cancer
In the era of genomic sequencing rapid strides have been made in understanding the molecular underpinnings of non-small cell lung cancer (NSCLC) and resulted in the development of highly effective therapies targeting specific molecular subsets of the disease. Response rates (RR) have increased dramatically from ~30% with cytotoxic chemotherapy to greater than 60% with biologic therapies such as erlotinib in patients with tumors harboring specific molecular alterations. However, despite the impressive activity of targeted therapy, durable responses are uncommon and tumors tend to develop resistance to treatment within months. Thus, there is a pressing need for the development of newer modalities of treatment for advanced lung cancer.
Immunotherapy offers the hope of overcoming the inherent weaknesses of conventional systemic therapies by initiating or amplifying an effective anti-tumor immune response. In recent years tremendous changes have occurred to the landscape of immunotherapy for solid tumors. Various facets of the immune system have been targeted to break immunological tolerance induced by tumors and resulted in the development of therapeutic vaccines against tumor antigens, adoptive T-cell therapies and antibodies designed to block immune checkpoints that result in T cell anergy. In the pre-checkpoint blockade era lung cancer had been considered a poorly immunogenic tumor and results of immunotherapy had been generally disappointing (1). However, targeting of cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programed death 1 (PD-1) or its ligand (PD-L1) has resulted in potentially practice-changing observations of safety coupled with impressive and durable anti-tumor activity.
A subgroup analysis of a phase I study of nivolumab in patients with NSCLC presented by Brahmer and colleagues at the 2014 annual meeting of the American Society of Clinical Oncology provides a snapshot of the potential benefits of targeting PD-1 in patients with NSCLC (2). A total of 129 patients with previously treated NSCLC were enrolled in a large expansion cohort of this study to assess the clinical activity of nivolumab at doses of 1, 3 and 10 mg/kg. Median overall survival (OS) was 9.9 months, RR was 17% and the median duration of response (DOR) was 17 months (Table 1). Clinical activity was seen in all patient subgroups independent of histology, number of prior lines of therapy, mutational status of NSCLC and PD-L1 expression on tumor cells.
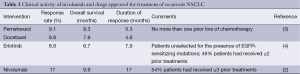
Full table
Drugs approved for treatment of recurrent NSCLC include pemetrexed, docetaxel and erlotinib. Results of clinical trials evaluating these drugs when compared with nivolumab in the setting of previously treated, advanced NSCLC show that a larger fraction of more heavily pretreated patients appear to benefit from nivolumab with higher RR, more durable responses and longer survival (Table 1). Additionally, Brahmer et al. (2) show that treatment with nivolumab is better tolerated with grade 3-4 adverse events (AE) observed in 14% patients. In comparison, the frequency of grade 3-4 neutropenia alone was 40% with docetaxel and 5% with pemetrexed and a significant proportion of patients underwent hospitalization for neutropenic fever (13% with docetaxel and 2% with pemetrexed) or for other drug-related AEs (11% with docetaxel and 6% with pemetrexed).
The clinical activity of nivolumab in patients with previously treated NSCLC also compares favorably with standard therapy for patients earlier in the disease course with untreated, inoperable, advanced or recurrent NSCLC. The phase III AVAPERL study evaluated induction therapy with four cycles of bevacizumab (7.5 mg/kg), cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) followed by maintenance bevacizumab (7.5 mg/kg) or bevacizumab plus pemetrexed (7.5 mg/kg; 500 mg/m2) in patients with non-squamous NSCLC who had responded to or had disease stabilization with induction therapy (5). The OS was 15.9-19.8 months from induction and 13.2-17.1 months from randomization to maintenance therapy. The 1- and 2-year OS rates were 68-72% and 34-40% respectively. The EURTAC study evaluated erlotinib for frontline treatment of EGFR-mutated NSCLC (6). Although OS was 19.3 months, the DOR was only 8.2 months. In comparison, treatment with nivolumab at a dose of 3 mg/kg in heavily pre-treated patients is associated with median OS of 14.9 months, 1- and 2-year OS of 56% and 45% respectively, and an estimated median DOR of 74 weeks (17.3 months). Thus, for those patients who respond, the durability of the response is what sets this approach apart from prior therapeutic strategies.
With the rapid development of PD-1/PD-L1 inhibitors there is a pressing need to develop predictive biomarkers to identify patients most likely to respond to treatment. The Brahmer study showed a RR of 15% in PD-L1 expressing tumors (at least 5% tumor cells showed membranous PD-L1 staining using the Dako immunohistochemistry (IHC) assay utilizing clone 28-8) and 14% in PD-L1-negative tumors. However, with a 1% cut-off for PD-L1 expression, the RR in PD-L1-positive and negative tumors was 13% and 17% respectively. This is in contrast to observations by Garon et al. who showed a RR of 24% in PD-L1-positive tumors (≥1% tumor PD-L1 expression with a prototype IHC assay using the 22C3 antibody) versus 8% in PD-L1-negative tumors (7). These results highlight the need for developing standardized assays and threshold criteria to determine PD-1/PD-L1 expression before using it as a selection criterion for enrollment on clinical trials evaluating anti-PD-1/PD-L1 antibodies. Since a fairly large proportion of patients with PD-L1 negative tumors also derives benefit from PD-L1 inhibition, it is imperative to understand the role of other biologic variables like the tumor microenvironment that could potentially determine sensitivity to checkpoint inhibition (8). The role of somatic mutations in determining response to checkpoint inhibitors in NSCLC also needs further evaluation. It has been observed that tumors with a high frequency of somatic mutations such as melanoma and NSCLC are more likely to respond to anti-PD-L1 therapy (9). Among patients with NSCLC, the mutational rate is higher in current or former smokers compared to never smokers and the former have been observed to have higher RR to anti-PD-L1 inhibition than the latter. It is also important to realize that not all mutations are immunologically relevant and have an ability to mediate tumor rejection. The presence of certain epitopes (rejection antigens) may play a more important role in influencing the ability of tumors to respond to checkpoint inhibitors than the mutational frequency itself.
Another important observation from the study by Brahmer et al. (2) is the relationship between the dose of nivolumab and OS. The longest median survival of 14.9 months and the highest 1- and 2-year survival rates of 56% and 45% respectively were noted at a dose of 3 mg/kg with an apparent reduction in median survival to 9.2 months at a dose of 10 mg/kg. Although only 59 patients were treated at a dose of 10 mg/kg compared to 37 patients at 3 mg/kg, and these cohorts were not randomized, the differences observed suggest caution when assuming a higher dose will lead to better activity with biologic agents. Higher RR and greater survival at dose levels lower than the highest dose level evaluated have been observed in trials of pembrolizumab in advanced, untreated PD-L1-expressing NSCLC (10) and nivolumab in advanced melanoma (11). These observations merit further evaluation to determine the optimal biological dose of immunotherapies such as checkpoint inhibitors.
The promising results obtained with nivolumab in advanced, previously treated NSCLC have spawned the development of several clinical trials which have the potential to generate practice-changing data. Nivolumab has been evaluated against docetaxel in previously treated NSCLC in two phase III trials that have completed accrual (NCT01673867 and NCT01642004) with results expected soon. Nivolumab is also being evaluated against cytotoxic chemotherapy in the front-line setting in a phase III trial (NCT02041533). An ongoing phase I trial is being conducted to determine the safety and tolerability of adding nivolumab to various treatment regimens including chemotherapy, bevacizumab, ipilimumab and erlotinib in patients with advanced lung cancer (NCT01454102). Secondary endpoints of this study include determination of the objective response rate and the progression free survival rate. The addition of nivolumab to ipilimumab aims to take advantage of the phenomenon of immunologic intensification with a goal of generating deeper and more durable responses while minimizing toxicity. Ongoing phase I trials of nivolumab in combination with anti-killer immunoglobulin receptor (KIR) antibodies and lymphocyte activation gene 3 (LAG-3) inhibitors will also help in determining the benefit of immunological intensification in NSCLC (NCT01968109 and NCT01714739). Future studies need to focus on the role of nivolumab as part of multimodality therapy in the neoadjuvant and adjuvant setting for patients with early-stage or locally advanced NSCLC.
Acknowledgements
The authors acknowledge the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research for their support of this study.
Disclosure: The authors declare no conflict of interest.
References
- Rolfo C, Sortino G, Smits E, et al. Immunotherapy: is a minor god yet in the pantheon of treatments for lung cancer? Expert Rev Anticancer Ther 2014;14:1173-87. [PubMed]
- Brahmer JR, Horn L, Gandhi L, et al. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): Survival and clinical activity by subgroup analysis. J Clin Oncol 2014;32:abstr 8112.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [PubMed]
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32. [PubMed]
- Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol 2014;25:1044-52. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Garon EB, Leighl NB, Rizvi NA, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8020.
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014;20:5064-74. [PubMed]
- Champiat S, Ferté C, Lebel-Binay S, et al. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology 2014;3:e27817. [PubMed]
- Rizvi NA, Garon EB, Patnaik A, et al. Safety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8007.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [PubMed]


