
Comparison of robotic-assisted lobectomy with video-assisted thoracic surgery for stage IIB–IIIA non-small cell lung cancer
Introduction
Minimally invasive surgery (MIS), including video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracic surgery (RATS), has been widely performed for the treatment of early stage non-small cell lung cancer (NSCLC). Compared with conventional thoracotomy, MIS is associated with reduced postoperative pain, faster recovery, and fewer complications (1-3). Generally, minimally invasive lung resection is indicated for early stage NSCLC, and with more clinical experience being gained in MIS and the rapid development of surgical technics and instruments, such as endoscopic staplers with rotating heads, high-definition 3D cameras, more and more locally advanced NSCLC patients are being treated by the minimally invasive approach. For appropriately selected patients, previous reports have confirmed that both RATS and VATS are feasible and acceptable in morbidity and mortality along with being associated with similar long-term outcomes compared with thoracotomy (4-9).
Robot-assisted surgery has several advantages over VATS, including 3D visibility and mechanical wrists which enable more bend and rotation than the human hand (10,11). Thus, complete lymph node dissection is easier to perform with RATS than with VATS (12), and so RATS is especially suitable for lung cancer cases with lymph node involvement. However, there are no currently published series comparing these two minimally invasive approaches specifically for locally advanced NSCLC. Therefore, we conducted this study to compare both short- and long-term outcomes of patients who underwent RATS or VATS lobectomy for stage IIB–IIIA (N1–2) NSCLC.
Methods
Patient selection
We performed a retrospective review of the prospectively collected database of our hospital between January 2014 and January 2017. The study was approved by institutional ethics committee of Shanghai Chest Hospital (No. KS1735). Eligible criteria were patients who underwent robotic or VATS lobectomy for clinical stage IIB–IIIA (N1–2) NSCLC by the eighth American Joint Committee on Cancer (AJCC 8) staging system (13). Patients with no lymph node involvement or who had undergone wedge resection or segmentectomy were excluded. Patients who had small cell lung cancer or incomplete data were also excluded. In all, 121 patients were included, of whom 36 had a robotic lobectomy and 85 had a VATS lobectomy. All the patients underwent routine serum chemistry, cardiopulmonary function testing, contrast-enhanced thoracic computed tomography (CT), positron emission tomography, brain magnetic resonance imaging or CT. Endobronchial ultrasound-transbronchial needle aspiration or mediastinoscopic biopsy was not mandatory in every patient.
Surgical procedures
Under general anesthesia, patients were placed on double-lumen tracheal intubation and contralateral single-lung ventilation. For both VATS and robotic lobectomy, 4 incisions including a 4–5 cm main incision in the 4th intercostal space at the site of the anterior axillary line were created without rib spreading. The surgical procedures for the pulmonary vessels and bronchi were individually performed, as previously reported (14). All patients underwent systematic mediastinal lymph nodal dissection; lymph nodes in groups 2, 4, 7, and N1 were resected for the right-side tumor, and lymph nodes in groups 5, 6, 7, and N1 were resected for the left-side tumor. Conversion was defined as the use of a rib-spreading thoracotomy at any point during the surgery.
Statistical analysis
Categorical data are summarized as numbers and percentages. Continuous data are summarized as means with standard deviations (SDs). Differences between the RATS and VATS groups were explored using Pearson’s chi-square test for categoric data and Student t test or Mann–Whitney U test for continuous variables. Since the distribution of age, sex, smoking status, tumor size, pulmonary function, clinical stages were comparable between the two groups (Table 1), we did not perform propensity score matching in further analysis. Overall survival (OS) was calculated from the day of surgery to the time of death. Disease-free survival (DFS) was calculated from the day of surgery to the date of cancer recurrence or death from any cause. OS and DFS were evaluated by Kaplan–Meier survival analysis. Log-rank tests were used to determine the statistical significance of survival between the two groups. Multivariate Cox regression models were built using factors with P<0.20 in univariate analyses. A two-sided P<0.05 was considered to indicate statistical significance. We used SPSS for Windows version 22 (IBM, Chicago, Il, USA) for statistical analysis.
Full table
Results
Patient characteristics
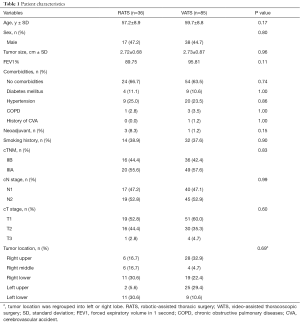
In total, 121 patients fit the inclusion criteria in this study, including 36 patients who underwent RATS lobectomy and 85 patients who underwent VATS lobectomy. Patient characteristics are shown in Table 1. The distribution of age, sex, smoking status, tumor size, pulmonary function, comorbidities, and clinical stage were comparable between the two groups.
Perioperative outcomes
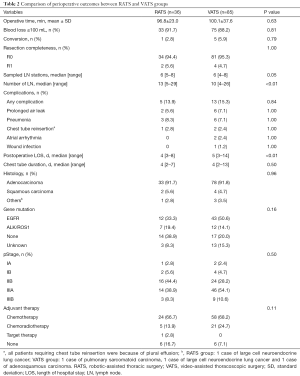
The comparison of perioperative outcomes of the two groups can be seen in Table 2. Operative time, blood loss, resection completeness, sampled lymph nodes stations, complications, chest tube duration, and final pathology stage were not significantly different between the two groups. There was no perioperative death in both groups. One patient (2.8%) in RATS group and 5 patients (5.9%) in the VATS group were converted to thoracotomy with no statistically significant difference. Reasons for conversion were severe adhesion (n=4) and lymph node calcification (n=2). Robotic lobectomy was associated with a shorter length of postoperative hospital stay (4 vs. 5 d, P<0.01) and more counts of lymph nodes harvested (13 vs. 10, P<0.01). For the majority of these patients who were stage II and stage III disease, adjuvant chemotherapy or chemoradiotherapy were recommended and the distribution of adjuvant therapy was comparable between the two groups.
Full table
Long-term outcomes
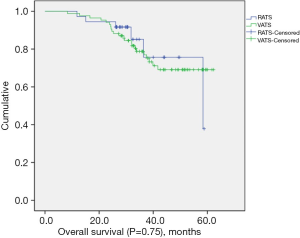
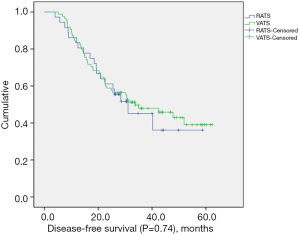
The median follow-up length was 33.9 months (range, 8.3–62.3 months). Two patients (2.4%) were lost to follow-up in the VATS group. The recurrence rate was 52.8% (n=19) in the RATS group and 54.1% (n=46) in the VATS group. Mortality from any cause was 16.7% (n=6) in the RATS group and 25.9% (n=22) in the VATS group. The median DFS for the RATS and VATS groups were 31.1 and 33.8 months, respectively. The corresponding 3-year DFS was 40.3% in the RATS group and 47.6% in the VATS group. The 3-year OS was 75.7% in the RATS group and 77.0% in the VATS group. There was no significant difference in OS (P=0.75) (Figure 1) and DFS (P=0.74) (Figure 2) between the two groups.
Multivariable Cox regression analysis of OS showed that two independent predictors were pathologic stage and gene mutation status (Table 3). Patients with advanced pathologic stage had a higher risk of death [hazard ratio (HR), 3.45; 95% confidence interval (CI), 1.36–8.80; P=0.01], while patients with gene mutation (EGFR/ALK/ROS1) had better OS (HR, 0.40; 95% CI, 0.18–0.90; P=0.03). No factors were associated with DFS in univariate or multivariate analysis (Table 4).
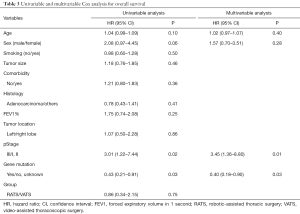
Full table
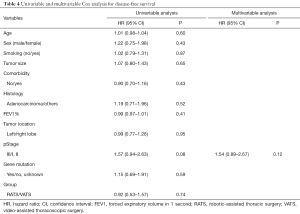
Full table
Discussion
MIS for the treatment of early stage NSCLC has rapidly spread during the last decade as a consequence of numerous investigators having proven its safety and effectiveness (15,16). Although the treatment of locally advanced NSCLC via minimal approaches is controversial, more and more studies have demonstrated that MIS, both VATS and RATS, are acceptable for selected patients (6,17,18). A propensity matched comparison of VATS with thoracotomy for locally advanced NSCLC by Chen et al. showed that video-assisted thoracoscopic lobectomy can be performed in the majority of cases without compromising perioperative outcomes and oncologic efficacy (19). In the largest series of patients with pN2 disease treated robotically (9), the conversion rate and complications were low while the survival was similar to that reported for open surgery. Even for bronchial sleeve resection, robotic surgery had similar short-term and mid-term outcomes compared with open procedures (20).
Compared with conventional VATS, robotic lung resection has been proposed to improve surgical outcomes by decreasing pain and enhancing recovery. A meta-analysis of 14 studies including 7,438 patients confirmed that RATS is a feasible and safe alternative to VATS with lower 30-day mortality and conversion rate (21). For long-term outcomes, a propensity matched comparison using the Memorial Sloan Kettering Cancer Center (MSKCC) database revealed a statistically significant difference between the robotic and VATS groups (22), but further multivariate analysis found that the surgical approach was not an independent predictor for OS or DFS. However, the majority of these studies focused on earl stage NSCLC, whereas in our study, we specifically compared these two minimally invasive approaches for stage IIB–IIIA (N1–2) patients. Our data showed OS and DFS were comparable between the two groups, and robotic lobectomy resulted in shorter postoperative length of stay and greater lymph node assessment, suggesting that the robotic approach is safe and effective in selected NSCLC patients with lymph node involvement.
In the current study, only 1 patient (2.8%) in the RATS group and 5 patients (5.9%) in the VATS group were converted to open surgery which was not greater than that in previous report (23), and no conversion occurred due to bleeding. The meta-analysis by Liang et al. (21) showed the conversion rate to open surgery was significantly lower in patients who underwent RATS compared to those who underwent VATS [10.3% vs. 11.9%; odds ratio, 0.57; P<0.001]. However, we did not see any difference between the two groups (2.8% vs. 5.9%; P=0.79).
Only a few patients in our series received neoadjuvant therapy, because about half of these patients were N1 disease. However, for the remaining 64 patients with suspected or proven N2 disease, only 4 patients received inductive chemotherapy. The reason for this is that in our center, we tend to choose surgery followed by chemotherapy or chemoradiotherapy for the treatment of stage IIIA NSCLC if the lymph nodes are smaller than 3 cm.
Lymphadenectomy plays an important role in the treatment of lung cancer, not only by clearing metastatic lesions, but also providing the precise stage assessment to decide whether adjuvant therapy is needed. A previous report showed a greater number of examined lymph nodes were associated with more accurate node staging and better long-term survival (24). The comparison of lymphadenectomy between robotic and VATS lobectomy varied from study to study. Toker et al. (12) and Yang et al. (17) published their study comparing RATS, VATS, and thoracotomy and found that RATS dissected more lymph nodes at the N1 level and harvested a higher number of median stations of lymph nodes. Wilson et al. (25) investigated the nodal upstaging rate of early stage NSCLC patients who underwent robotic and VATS lung resection and reported a rate of nodal upstaging for robotic resection appeared to be superior to VATS and similar to the thoracotomy data. However, another study conducted by Lee et al. (26) showed the two approaches were similar in node upstaging for clinical N0 lung cancer. Moreover, Louie et al. (27) and Lee et al. (28) also compared RATS and VATS and concluded that both approaches were similar to lymph node dissection. In our study, we found robotic lobectomy was associated with more counts of lymph nodes (13 vs. 10, P<0.01); however, greater lymph node assessment did not translate into survival benefit.
The treatment for NSCLC has been refined in recent years with treatments allocated according to histology and specific molecular features. Tyrosine kinase inhibitors (TKIs) can significantly prolong OS for patients with advanced NSCLC harboring epidermal growth factor receptor (EGFR)-mutation, anaplastic lymphoma kinase (ALK)-rearrangement, or c-ros oncogene 1 receptor kinase (ROS1) fusion (29-31). In our series, multivariable Cox regression analysis revealed patients harboring theses three driver mutations had better over survival (HR, 0.40; 95% CI, 0.18–0.90; P=0.03). A comprehensive investigation of oncogenic driver mutations in Chinese NSCLC patients included 1,356 resected lung adenocarcinomas showed the frequency of EGFR, ALK, and ROS1 were 63.1%, 5.2%, and 0.8%, respectively (32). In the current study, the frequency of EGFR was 33.3% in the RATS group and 50.6% in the VATS group; meanwhile, the frequency of ALK/ROS1 was 19.5% in the RATS group and 14.1% in the VATS group which was higher than that in previous reports because of the small sample size.
Our study has several limitations. First, the analysis was performed retrospectively, and selection bias, which might have influenced the outcomes, could not be avoided. Second, the sample size of the two groups were relatively small and can only reflect the experience of a single center. Third, we did not have the detailed data of adjuvant chemotherapy regimens or further treatment after relapse which might have affected conclusions about DFS and OS. A future multicenter randomized clinical trial is warranted to further compare these two minimally invasive approaches.
Conclusions
To our knowledge, this is the first study specifically comparing robotic surgery with VATS for the treatment of stage IIB–IIIA NSCLC. Our findings suggest that for selected NSCLC patients with lymph node involvement, robotic lobectomy is safe and effective with a low complication rate and similar long-term outcomes compared with VATS lobectomy. Furthermore, the robotic approach resulted in shorter postoperative length of stay and greater lymph node assessment.
Acknowledgments
Funding: This work was supported by Shanghai Shen Kang Eighth Batch of “Emerging Frontier” Projects (SHDC12016113).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Institutional Ethics Committee of Shanghai Chest Hospital (No. KS1735).
References
- Farivar AS, Cerfolio RJ, Vallieres E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innovations (Phila) 2014;9:10-5. [Crossref] [PubMed]
- Suda K. Intraoperative molecular imaging—a bright navigator for thoracic surgeons in the era of limited resection. Transl Lung Cancer Res 2018;7:S232-5. [Crossref] [PubMed]
- Bendixen M, Jorgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Gonfiotti A, Bongiolatti S, Bertolaccini L, et al. Thoracoscopic lobectomy for locally advanced-stage non-small cell lung cancer is a feasible and safe approach: analysis from multi-institutional national database. J Vis Surg 2017;3:160. [Crossref] [PubMed]
- Hennon MW, Demmy TL. Video-assisted thoracoscopic surgery (VATS) for locally advanced lung cancer. Ann Cardiothorac Surg 2012;1:37-42. [PubMed]
- Park BJ, Yang HX, Woo KM, et al. Minimally invasive (robotic assisted thoracic surgery and video-assisted thoracic surgery) lobectomy for the treatment of locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:S406-13. [Crossref] [PubMed]
- Petersen RP, Pham D, Toloza EM, et al. Thoracoscopic lobectomy: a safe and effective strategy for patients receiving induction therapy for non-small cell lung cancer. Ann Thorac Surg 2006;82:214-8; discussion 219. [Crossref] [PubMed]
- Tian Z, Sui X, Yang F, et al. Is video-assisted thoracoscopy a sufficient approach for mediastinal lymph node dissection to treat lung cancer after neoadjuvant therapy? Thorac Cancer 2019;10:782-90. [Crossref] [PubMed]
- Veronesi G, Park B, Cerfolio R, et al. Robotic resection of Stage III lung cancer: an international retrospective study. Eur J Cardiothorac Surg 2018;54:912-9. [Crossref] [PubMed]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236-42; discussion 242-4. [Crossref] [PubMed]
- Demir A, Ayalp K, Ozkan B, et al. Robotic and video-assisted thoracic surgery lung segmentectomy for malignant and benign lesions. Interact Cardiovasc Thorac Surg 2015;20:304-9. [Crossref] [PubMed]
- Toker A, Ozyurtkan MO, Demirhan O, et al. Lymph Node Dissection in Surgery for Lung Cancer: Comparison of Open vs. Video-Assisted vs. Robotic-Assisted Approaches. Ann Thorac Cardiovasc Surg 2016;22:284-90. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Huang J, Luo Q, Tan Q, et al. Initial experience of robot-assisted thoracoscopic surgery in China. Int J Med Robot 2014;10:404-9. [Crossref] [PubMed]
- Boffa DJ, Kosinski AS, Furnary AP, et al. Minimally Invasive Lung Cancer Surgery Performed by Thoracic Surgeons as Effective as Thoracotomy. J Clin Oncol 2018;36:2378-85. [Crossref] [PubMed]
- Dziedzic R, Marjanski T, Binczyk F, et al. Favourable outcomes in patients with early-stage non-small-cell lung cancer operated on by video-assisted thoracoscopic surgery: a propensity score-matched analysis. Eur J Cardiothorac Surg 2018;54:547-53. [PubMed]
- Yang CF, Meyerhoff RR, Mayne NR, et al. Long-term survival following open versus thoracoscopic lobectomy after preoperative chemotherapy for non-small cell lung cancer. Eur J Cardiothorac Surg 2016;49:1615-23. [Crossref] [PubMed]
- Veronesi G, Novellis P, Difrancesco O, et al. Robotic assisted lobectomy for locally advanced lung cancer. J Vis Surg 2017;3:78. [Crossref] [PubMed]
- Chen K, Wang X, Yang F, et al. Propensity-matched comparison of video-assisted thoracoscopic with thoracotomy lobectomy for locally advanced non-small cell lung cancer. J Thorac Cardiovasc Surg 2017;153:967-76.e2. [Crossref] [PubMed]
- Gu C, Pan X, Chen Y, et al. Short-term and mid-term survival in bronchial sleeve resection by robotic system versus thoracotomy for centrally located lung cancer. Eur J Cardiothorac Surg 2018;53:648-55. [Crossref] [PubMed]
- Liang H, Liang W, Zhao L, et al. Robotic Versus Video-assisted Lobectomy/Segmentectomy for Lung Cancer: A Meta-analysis. Ann Surg 2018;268:254-9. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Agzarian J, Fahim C, Shargall Y, et al. The Use of Robotic-Assisted Thoracic Surgery for Lung Resection: A Comprehensive Systematic Review. Semin Thorac Cardiovasc Surg 2016;28:182-92. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg. 2014;97:1901-6; discussion 1906-7.
- Lee BE, Shapiro M, Rutledge JR, et al. Nodal Upstaging in Robotic and Video Assisted Thoracic Surgery Lobectomy for Clinical N0 Lung Cancer. Ann Thorac Surg 2015;100:229-33; discussion 233-4. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Lee BE, Korst RJ, Kletsman E, et al. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: are there outcomes advantages? J Thorac Cardiovasc Surg 2014;147:724-9. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Wang R, Zhang Y, Pan Y, et al. Comprehensive investigation of oncogenic driver mutations in Chinese non-small cell lung cancer patients. Oncotarget 2015;6:34300-8. [PubMed]


