
Peritoneal mesothelioma
Introduction
Malignant peritoneal mesothelioma (MPM) is a rare and lethal cancer arising from the mesothelial lining of the peritoneum. Approximately 500 to 700 new cases are diagnosed annually in the United States (1-3). The disease is largely considered chemotherapy resistant although without treatment, median overall survival (OS) is 6 months to 1 year (1,2). Pleural mesothelioma is more common than the peritoneal variant which represents 15–20% of new cases of mesothelioma (2). The disease can afflict patients across a broad age range however, the mean age at diagnosis is 64 years, (1). Patients present with nonspecific symptoms and usually with diffuse intraperitoneal disease. MPM is equally distributed in men and women, compared to pleural mesothelioma which demonstrates predominance in men (4). Over 90% of patients diagnosed with MPM in the United States are non-Hispanic white, with black patients being the second most commonly affected group (4.6%) (5). Two hallmark features of MPM include (I) the variability of tumor aggressiveness and disease progression and (II) the tendency of the disease to remain within the peritoneal cavity. Morbidity and mortality of the disease are almost always related to local and regional effects of tumor burden within the abdomen, mainly small bowel obstruction and cancer cachexia (2,6). The standard of care of MPM remains cytoreductive surgery (CRS) to aggressively remove the tumors, followed by hyperthermic intraperitoneal chemotherapy (HIPEC) to destroy microscopic residual cancer cells (3,7). With advances in CRS-HIPEC over the last decades, the procedure has evolved from a palliative endeavor to being performed with curative intent with median OS in some series reaching 5 years (8-10).
Risk factors
The first reported case of MPM was in a 35-year-old male miller in 1908 (11). Since that time, there have been major advances into understanding the etiology of the disease including environmental exposure and germline genetic alterations that increase the risk of developing the disease. The correlation of mesothelioma to environmental asbestos was first elucidated in the mid-1960s when epidemics in asbestos miners and shipyard workers were exposed (12-14). However, cases of MPM have been linked less frequently to asbestos exposure compared to the pleural form, with as few as 8% of patients identifying previous exposure in MPM compared to greater than 80% in pleural mesothelioma (15-17). In addition, MPM occurs usually around 20 years after exposure vs. 30–40 years for pleural variants (1). Other mineral fibers likely play a causative role, with the silicate fiber erionite also being potent inducer of MPM (12,18). Other factors described include therapeutic radiation, Thorotrast dye historically used in angiographic studies, papovavirus, simian virus and chronic inflammation (18). In 2019 asbestos remains the most identifiable risk factor in MPM, though epidemiologic projections recently published state that beyond the year 2040 asbestos will not be linked to new cases of mesothelioma diagnosed in the United States (13). Therefore, other causes remain to be discovered.
Outcomes and survival
CRS-HIPEC is the only treatment that appears to meaningfully impact the natural history of MPM. Table 1 summarizes the largest studies performed to date, demonstrating improved survival with CRS-HIPEC. A 2009 multi-institutional study by Yan et al. of 405 MPM patients undergoing CRS-HIPEC demonstrated a median OS of 53 months and 5-year OS of 47% (19). A 2013 study from three major referral centers showed 211 patients had a 5-year OS of 41% and 10-year survival of 26% after CRS-HIPEC (20). Reported OSs in patients undergoing CRS has also appeared to increase over time most likely due to better patient selection and decreased morbidity from the operative procedure (1). A SEER study of 1,591 patients with MPM noted OS improved to 38 months in 2006–2010 vs. 15 months in the 1991–1995 interval (P=0.01) (5). Factors associated with shortened survival include male sex, advanced age (>60 years), high grade (biphasic or sarcomatoid) histology, and large burden of disease at presentation (5). Surgical intervention, when possible, is independently associated with improved survival, related to the completeness of cytoreduction and administration of HIPEC (19). Unfortunately, up to 60% of patients may not receive surgery when diagnosed with MPM (5). Additional factors independently associated with improved outcome is favorable epithelioid histologic subtype, absence of lymph node metastases (19).

Full table
Histologic variants
MPM includes three histologic subtypes, the determination of which is crucial to guide management and understand biologic aggressiveness. Epithelioid subtype MPM (75–90% of cases) offers the best prognosis and resembles normal mesothelial cell histology with a predominantly tubulopapillary or trabecular pattern. Sarcomatoid subtypes show tightly arranged spindle cells, with malignant appearing osteoid, chondroid or muscular features. Biphasic subtypes contain both epithelioid and sarcomatoid elements, with at least 10% histologic features of each subtype present (16,21). Epithelioid subtypes uncommonly demonstrate mitotic figures. However, variants may demonstrate variable survival based on nuclear features and mitotic rate, with one study demonstrating high-risk features to be associated with a 21% 5-year survival vs. 57% 5-year survival with low-risk features (4). In a study of 64 tumors treated with CRS, poorly differentiated tumors showed an increased propensity for increased depth of invasion. The degree of tissue invasion correlated with tumor necrosis, nuclear grade and mitotic rate, but not increased tumor burden and distribution (21). Additional rare variants include pleomorphic, deciduoid, small and clear cell (15).
In this review, current knowledge about the diagnosis, treatment and outcomes of MPM are discussed, as well as future medical and surgical therapeutic approaches.
Clinical presentation and diagnosis
Patient presentation
Patients with MPM present with vague signs and symptoms, and most commonly report abdominal pain and increasing abdominal girth secondary to ascites (1,22). Other complaints may include weight loss, dyspnea, chest pain or a palpable abdominal mass. Average time from symptom onset to diagnosis of MPM is 4–6 months (4). Approximately 8% of patients are diagnosed incidentally with abdominal imaging or surgery performed for an unrelated indication (16). Upper endoscopy, colonoscopy and assessment of pelvic structures in women should be performed to rule out a gastrointestinal or gynecologic source of peritoneal disease (6).
Radiologic assessment
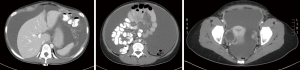
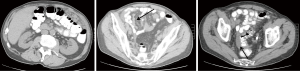
Computed tomography (CT) is the preferred first-line imaging modality. MPM deposits appear as intravenous contrast enhancing heterogeneous soft tissue masses with irregular margins. Differential diagnosis includes peritoneal carcinomatosis resulting from adenocarcinoma of gastrointestinal or ovarian origin, and more rarely lymphomatosis or tuberculous peritonitis (16). CT findings consistent with MPM include cases where no primary lesion is identifiable, no lymphadenopathy is noted, and the distribution of intra-abdominal masses is diffuse, many times with omental caking or thickening of the peritoneum. Presence of bicavitary disease extending into the mediastinum or thoracic pleura should be ruled out (7). Favorable CT findings include minimal soft tissue masses with ascites and normal small bowel and mesenteric anatomy (Figure 1). Unfavorable findings include the absence of ascites, hydroureter, tumors ≥5 cm in the lesser omentum, subpyloric space or jejunal regions, mesenteric or para-aortic lymphadenopathy, large and diffuse nodular thickening of all peritoneal surfaces with anatomic distortion of the bowel (3,23) (Figure 2). CT scan findings of MPM with bowel obstruction are especially worrisome (2). The role for positron emission tomography (PET)/CT in the initial diagnosis of MPM is limited (15).
Cytologic diagnosis and biomarkers
Cytologic sampling of ascites for diagnosis is difficult, as the fluid will contain a low number of malignant cells and results are often inconclusive (4). Definitive diagnosis usually requires CT-guided core needle biopsy or biopsy via diagnostic laparoscopy (3). Laparoscopic examination can confirm the extent of tumor burden and assess the feasibility of a complete CRS. However, caution should be taken via this approach, considering that laparoscopy may also underestimate extent of disease. Port sites should be limited, if possible, to the midline abdomen (linea alba) so tumor deposits can be excised during CRS-HIPEC. Adequate tissue sampling allows for assessment of invasion through the peritoneum into the underlying stroma and fat, which signify more aggressive biology and poorer prognosis (Figure 3) (24).
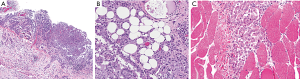
Histologic diagnosis of MPM should be performed by an expert pathologist, as specific evaluation of histologic subtype and invasiveness (including a high Ki-67 index and high mitotic rate) are essential to guide treatment recommendations (7). Performance of tumor immunohistochemistry (IHC) and employing a panel of markers is imperative to differentiate MPM from adenocarcinoma and peritoneal serous carcinoma (1). The most sensitive IHC markers include calretinin (100%), Wilm’s tumor (WT-1, 94%), and cytokeratin 5/6 (89%) (15). Mesothelin and fibulin-3 are specific for mesothelioma (7). At least two mesothelioma and two carcinoma biomarkers should be examined to confirm a diagnosis of MPM (16). Positive antibody staining for cytokeratin 5/6, calretinin, WT-1, human mesothelial cell 1 (HBME-1), thrombomodulin, or mesothelin, and negative antibody staining for carcinoembryonic antigen (CEA), TTF1, B72.3, MOC 31, Ber-Ep4, LeuM1, or Bg8 support a pathologic diagnosis of MPM (6,16,25). In addition, IHC demonstration of loss of BAP1 expression, a tumor suppressor gene to be discussed further in this review, suggests a diagnosis of malignancy and rules out benign mesothelial lesions and ovarian serous tumors. BAP1 expression is lost in 50% of pleural mesothelioma and two-thirds of MPM, although is found in less than 1% of high-grade serous carcinomas (16). As of now, loss of nuclear BAP1 confirms the diagnosis of MPM but cannot yet guide prognosis. Measurement of CA-125 is not routinely used in diagnosis.
Staging and recurrence
The peritoneal carcinoma index (PCI) is a reproducible, systematic method of determining the distribution and burden of disease (26). The PCI can be estimated prior to surgery via CT imaging, however the most accurate approach is intraoperative assessment. The peritoneal cavity is divided into 13 regions, with 9 regions being numbered in clockwise fashion beginning at the right hemidiaphragm (the umbilicus is labeled region 0) and the small bowel supplying 4 regions (upper and lower regions of jejunum and ileum). The size of the largest lesions within each region is graded from no gross disease to extensive disease, with lesion size (LS)-0 being no visible tumor, LS–1 tumors up to 5 mm, LS–2 tumors up to 5 cm and LS–3 tumors greater than 5 cm. The scores are tallied to range between 0–39, with 0 representing complete cytoreduction and 39 extremely high burden of disease.
Tumor, nodal and metastasis (TNM) staging has not historically been applied to MPM as the disease usually presents diffusely throughout the abdominal cavity and nodal or extraperitoneal spread is rare. However, Yan et al. proposed a TNM staging method guided by correlating the T stage to PCI quartiles. The PCI was stratified from 1–10, 11–20, 21–30, and >30, which corresponded to T1–T4 staging (27). Stage I (T1N0M0), stage II (T2N0M0 or T3N0M0), and stage III (T4, N1 and/or M1 disease) demonstrated a 5-year OS survival of 87%, 53% and 29% respectively.
Early recognition of MPM recurrence is imperative to early intervention. A recent study by Llanos et al. examined recurrence patterns in 130 patients with MPM who underwent CRS-HIPEC from a prospectively maintained database (28). The median time to recurrence was 14 months and 38 patients underwent 50 re-operations. The most common signs and symptoms of recurrence were abdominal pain (40%) and distention (34%). The most common radiologic finding was an abdominal mass (56%). Patients diagnosed with recurrence but without any signs or symptoms demonstrated an improved prognosis. The authors conclude that repeated CRS-HIPEC in select patients is associated with long term survival, although achievement of complete CRS is decreased in subsequent operations. Recommendations for surveillance included a 6-month postoperative visit for symptom review, an annual physical exam for 5 years, and bi-annual or annual CT with oral and intravenous contrast to detect radiologic recurrence. Biomarker testing is not routinely employed for surveillance, although if CA-125 levels are elevated at diagnosis, subsequent levels will be trended.
Surgical treatment and technique
The learning curve for CRS-HIPEC is steep, with an estimated 140 cases required to acquire expertise (29). The main determinant of MPM outcomes is the completeness of cytoreduction (15). Completeness of cytoreduction score (CC score) provides a uniform approach to describing the remaining tumor burden after surgical intervention. CC0 score signifies no visible residual disease, CC1 tumors measure <2.5 mm, CC2 tumors measure >2.5 mm and <2.5 cm, and CC3 is gross residual tumor measuring >2.5 cm. A CC score of 0 or 1 is considered successful CRS prior to HIPEC.
Patient selection for CRS/HIPEC
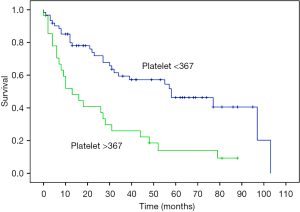
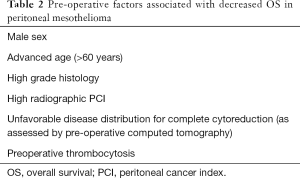
Favorable patient selection includes good performance status (frequently defined as ECOG 0 or 1), PCI and disease distribution favorable for complete or near-complete (CC0 or CC1) CRS, age <60 years, female sex, epithelioid histology, and absence of pretreatment thrombocytosis (Figure 4), (1,3,19,22,30). Factors associated with decreased OS are listed in Table 2. Decreased likelihood of CRS completion is associated with age >60 years, male sex, deep tissue invasion, solid tumor masses on the small bowel or adjacent mesentery on CT imaging, presence of ascites and pretreatment thrombocytosis (31). Patients with unfavorable histology (biphasic and sarcomatoid) are usually excluded from CRS-HIPEC and are referred for clinical trial or systemic chemotherapy (23). Contraindications for CRS-HIPEC include severe cardiac, hepatic or renal dysfunction, poor performance status (determined by ECOG status greater than 2 or surgeon assessment of frailty), extensive involvement of the small bowel or adjacent mesentery, extra-abdominal disease or para-aortic metastasis precluding the ability for complete CRS (32).
Full table
Biomarker testing and tumor histology are increasingly used to guide patient selection. A 2016 study by Kusamura et al. determined proliferation marker Ki-67 was an independent pre-CRS determinant of survival, with a Ki-67 ≤9% having a median OS of 86.6 months, and Ki-67 of >9% a median OS of 10.3 months, leading to the conclusion that these patients are unlikely to benefit from CRS-HIPEC (33). While biphasic histology has been historically defined as unfavorable for CRS-HIPEC, a 2018 retrospective analysis of the Peritoneal Surface Oncology Group International (PSOGI) registry examined outcomes in this subtype with further granularity (34). The analysis included 484 patients from 33 centers undergoing CRS-HIPEC with a CC0/1 score; 450 patients (93%) had epithelioid subtype, while 34 patients (7%) had biphasic mesothelioma. Patients achieving complete CRS (CC0), 5-year survival was 64.5 for epithelioid and 50.2% for biphasic patients (P=0.015). In procedures where tumor remained (CC2), median OS for patients with biphasic MPM was a dismal 4.3 months. The authors concluded that CRS-HIPEC should be offered for patients with biphasic histology and low volume disease amenable to complete cytoreduction.
Surgical approach
The widespread involvement of all parietal surfaces in MPM often requires extensive peritonectomy in order to achieve complete cytoreduction (16). Peritonectomy procedures have been described to fully address disseminated intraperitoneal disease; greater omentectomy-splenectomy, lesser omentectomy-cholecystectomy, with stripping of omental bursa, left upper and right upper quadrant peritonectomies, and pelvic peritonectomy with sleeve resection of the rectosigmoid colon if indicated. Tumor deposits involving the posterior porta hepatis and extensive involvement of the small and large bowel may not be amenable to CRS.
After CRS, HIPEC is performed in an attempt to address the remaining microscopic disease. Hyperthermia acts synergistically with intraperitoneal chemotherapic agents to deliver direct cytotoxic effects to the malignant cells, impair DNA repair, denature proteins, induce heat-shock proteins (HSP), induce apoptosis and inhibit angiogenesis (32). It is estimated cytotoxic agents can only penetrate up to a 3 mm depth, hence the importance of achieving a CC0 or CC1 resection (6). All accessible and enlarged lymph nodes should be sampled (23). In contrast to peritoneal spread of gastrointestinal malignancies, if CRS is not attainable in patients with MPM, the surgeon may consider proceeding with HIPEC as some evidence shows palliative benefit in patients with ascites (3).
HIPEC chemotherapy regimens
While the technique of CRS is fairly uniform, intraperitoneal chemotherapy regimens are not yet standardized. Overall, HIPEC regimens ideally should be the optimal method of delivering an effective chemotherapeutic agent to the peritoneal cavity resulting in penetration of individual tumor cells and cell death (35). A regimen increasingly represented in the literature is cisplatin, an alkylating agent causing apoptosis, which offers excellent augmentation with hyperthermia. This is usually supplemented with doxorubicin, an anthracycline antibiotic, which augments the action of cisplatin. Due to the high molecular weight, these agents have slow clearance from the peritoneal cavity and offer further opportunity for tumor cell penetration. However, mitomycin C is also widely used. Conclusions regarding which regimen is most efficacious are difficult secondary to the retrospective nature existing studies.
One such study was the 2018 RENAPE study from France, which retrospectively correlated various HIPEC agents with survival outcomes after CRS-HIPEC (36). No statistical differences were found in 211 patients with MPM receiving cisplatin, cisplatin plus doxorubicin, mitomycin C, oxaliplatin or oxaliplatin with irinotecan. The most common regimens were cisplatin and doxorubicin (n=60) and mitomycin C (n=52). However, OS was improved when using two agents vs. one agent [hazard ratio (HR), 0.54; 95% confidence interval (CI), 0.31–0.95; P=0.03] with no increases in postoperative morbidity. This held true for progression-free survival (PFS) as well (HR, 0.48; 95% CI, 0.21–1.07; P=0.07) in CC0 patients with epithelioid subtype. Another small retrospective study in 34 patients with MPM showed that patients who received cisplatin HIPEC were more likely to survive at 1, 2 and 3 years postoperatively than those receiving mitomycin C. While not statistically significant with a small cohort, cisplatin patients demonstrated median OS of 40.8 compared to 10.8 months with mitomycin C (P=0.22) (37).
Surgical risk and morbidity
CRS-HIPEC is a prolonged procedure secondary to the frequent need for multivisceral resections, bowel anastomoses, peritonectomies. In addition, HIPEC adds physiologic stress and metabolic demands from hyperthermia and regional delivery of cytotoxic agents. Complications may range from wound infections, urinary tract infection, pneumonia and prolonged ileus, to more life-threatening complications such as sepsis or hemorrhage. Long-term sequelae may include myelosuppression, bowel obstruction and enteric fistula. CRS-HIPEC has long been considered a procedure associated with the potential for severe morbidity although more recent reports suggest morbidity and mortality are decreasing. A recent analysis of the American College of Surgeons National Surgical Quality Improvement Project (ACS-NSQIP) by Foster et al. demonstrated that 1,822 patients undergoing CRS-HIPEC from 2005 to 2015 had comparable 30-day postoperative outcomes to other major abdominal oncologic operations such as pancreaticoduodenectomy, esophagectomy, or major partial hepatectomy (38). The authors conclude this trend is likely secondary to improved patient selection and increasing surgeon experience performing the procedure at high-volume centers.
Current areas of research
Surgical approaches
The two main methods of delivering intraperitoneal chemotherapeutic agents in the operating room are both described in the literature. The “open” (or “coliseum”) technique delivers HIPEC through an open peritoneal cavity. Most commonly, the skin overlying the laparotomy incision is sutured to a retractor ring above the wound. A plastic sheet covers the abdominal cavity and the surgeon’s hand is introduced through a defect in the sheet to allow for manual stirring of the peritoneal contents during HIPEC (32). The “closed” technique temporarily closes the laparotomy incision via a running skin suture while the abdomen is perfused. At this time, there is no proven superiority regarding safety or outcomes of one approach over another (3). The closed method offers several advantages including ease of use, with less spillage of chemotherapeutic agents and thus less exposure of the drug to operating room staff. While open vs. closed techniques have not been directly compared in humans, a murine model study in 2018 demonstrated some important potential differences. Eleven male rats received fixed-dose intraperitoneal pemetrexed at perfusion temperature of 40 °C for 25 minutes via open or closed techniques (39). Postoperative drug concentration in the portal blood, systemic blood and peritoneal tissue were measured. No differences in peritoneal tissue concentration were found (18.07 vs. 19.17 µg/g, P=0.51), though portal blood concentrations (93.17 vs. 52.50 µg/mL, P<0.001) and systemic blood concentrations (76.26 vs. 51.65 µg/mL, P<0.001), were significantly higher in the open technique group. This study raised the question of whether systemic toxicity could be increased with open HIPEC.
The potential effects of core body temperature (CBT) and increased abdominal pressure (IAP) during HIPEC have been recently examined. A 2019 study by Lemoine et al. randomized 31 patients with colorectal peritoneal metastases to either a body surface area (BSA) or concentration-based HIPEC (HIPEC-CONC) protocol in a phase III clinical pilot trial (29). HIPEC was performed using the open technique, and a fixed concentration of 460 mg/m2 in 2 L/m2 0.9% sodium chloride carrier solution was compared to dosing of oxaliplatin based on calculated BSA. Higher drug concentrations were found in tumor tissue samples with the HIPEC-CONC regimen. Although a significantly higher amount of oxaliplatin was given to patients in the HIPEC-CONC group, no major differences in postoperative morbidity were found. However, hospital length of stay was significantly increased in the HIPEC-CONC group (23 vs. 15 days in the BSA group; P=0.003). Long term results including survival were not examined in this study.
High IAP resulting in intra-abdominal hypertension, associated with the closed technique, has been theorized to cause increased complications secondary to decreased venous return and end-organ effects. However, this hypothesis may not hold true in clinical studies. A phase II randomized 2019 study by Kusamura et al. in 38 patients examined the effects of IAP during closed HIPEC on cisplatin uptake by tumor cells and short-term surgical outcomes (40). IAP was measured via intravesicular catheter intraoperatively. Median IAP in the high arm was 19 and 11 mmHg in the low arm. Interestingly, cisplatin concentrations did not differ in the residual tumor tissue and muscular fascia samples, however were increased in the mesenteric peritoneal samples in the high IAP group (5.4 vs. 2.7 ng/mg low IAP; P=0.048). Postoperative complications were not increased in the high IAP group. This study suggests the desired high intraperitoneal tissue concentrations are increased not only by hyperthermia augmentation but also with the addition of increased IAP. The method of manipulating IAP during HIPEC may partially attainable by controlling abdominal wall muscle relaxation or increasing perfusate volume. A retrospective 2018 study by Goldenshluger et al. of 115 patients undergoing CRS-HIPEC supported that postoperative complications are not more frequent with increased IAP (41). However, in multivariate analysis, elevated mean CBT was a positive predictor. In fact, each one degree increases of CBT doubled the likelihood of postoperative complications, and correlated to severity. Optimal CBT during HIPEC is being further explored, as low CBT could result in suboptimal HIPEC and early recurrence, while high CBT increases morbidity.
Adjuncts to intraoperative chemotherapy have also been explored. Early postoperative intraperitoneal chemotherapy (EPIC) entails delivery of chemotherapy from postoperative day 1 through day 4 or 5 through an inflow and outflow drain placed during initial surgery. Regimens include 5-flourouracil (5-FU) or paclitaxel which remain within the peritoneal cavity for up to 23 hours, with multiple cycles (35). The ability to deliver additional agents to the peritoneum prior to formation of postoperative adhesions could potentially offer more uniform drug distribution. Disadvantages of EPIC may include an increased risk of infection and postoperative complications. McConnell et al. compared patients receiving HIPEC with mitomycin C and EPIC with 5-FU for 5 days (n=85) vs. HIPEC with oxaliplatin and intravenous 5-FU with no EPIC (n=113) (42). This study included all patients with peritoneal metastases, including gastrointestinal sources and MPM. The rate of grade III complications was higher in the HIPEC + EPIC group (44.7% vs. 31.0%; P=0.05). On multivariate analysis, HIPEC + EPIC with a PCI >26 was associated with increased complications. The authors concluded that surgeons should consider using HIPEC only as evidence for addition of EPIC is still lacking.
Systemic chemotherapy
MPM demonstrates relative chemoresistance, and systemic therapy has not shown to be effective in terms of prolonged survival (36). Systemic chemotherapy may be offered to patients with unresectable MPM, patients who decline surgical intervention, those with biphasic or sarcomatoid high-risk histology, extra-abdominal disease, and those with poor performance status (32). Pemetrexed monotherapy has an estimated median OS of 8.7 vs. 13.1 months for pemetrexed and cisplatin combination therapy. Therefore, current standard is doublet therapy with a suggested regimen of pemetrexed 500 mg/m2 with cisplatin 75 mg/m2 administered every 21 days for at least 6 cycles (6). Contraindications for cisplatin therapy include renal dysfunction, bone marrow suppression and peripheral neuropathy. Pemetrexed should not be given to patients with renal dysfunction, thrombocytopenia, or if pregnant or lactating. Pemetrexed is well-tolerated with a low rate of adverse effects (43). A phase II clinical trial of pemetrexed and gemcitabine treatment showed inferior survival results and a response rate of 15%, median time to disease progression of 10.4 months and median OR of 26.8 months (44). The authors suggest gemcitabine is a safe alternative treatment to patients who cannot tolerate cisplatin, though this regimen is now relatively uncommon.
For resistant or relapsed disease, no second-line systemic therapies are currently recommended (43). Vascular endothelial growth factor (VEGF) inhibitor bevacizumab has been recently explored as a therapeutic adjunct to current regimens. The results of clinical trials are largely extrapolated from pleural mesothelioma research and applications for VEGF inhibitors in MPM is unknown. A phase III controlled open-label trial randomized 448 patients with pleural mesothelioma to intravenous pemetrexed and cisplatin, with or without bevacizumab (45). Median OS was longer in the bevacizumab group [18.8 (95% CI, 15.9–22.6) vs. 16.1 months (95% CI, 14.0–17.9); P=0.0167]. There was an increase in thrombotic events in the groups receiving the anti-VEGF agent. Some have expressed concerns the agent may increase complications if surgical intervention is eventually pursued, thus there is no current role for bevacizumab in the treatment of operable MPM (25).
The practice of neoadjuvant chemotherapy prior to CRS-HIPEC is variable throughout different centers. While further studies are warranted, current standard of care remains upfront CRS-HIPEC for operable disease, followed by a patient-tailored discussion regarding risks and benefits of adjuvant chemotherapy.
Genetic and molecular factors
BRCA associated protein 1 (BAP1) is a nuclear deubiquitinylase involved in DNA repair and apoptosis of DNA mutations (46). BAP1 localizes in both the nucleus and cytoplasm. BAP1 mutation or loss of BAP1 results in accumulation of genetic mutations, eventually leading to malignant transformation. Carriers of BAP1 mutations have 50% of normal BAP1 levels and a reduced ability to repair DNA after environmental insults such as asbestos exposure, ultraviolent light and radiation. In mesothelioma, it is unclear whether BAP1 alterations are causative, or simply lower the threshold of asbestos exposure required to cause malignancy (47) Mutations, detected by IHC, are present in approximately 27–67% of pleural mesotheliomas (17,48). Few studies have examined the prevalence of mutations in MPM, though a study by Singhi et al. of 86 patients undergoing CRS-HIPEC demonstrated loss of BAP1 protein expression in 49 patients (57%) (48). The relationship of mesothelioma to germline BAP1 mutations was initially discovered via studies of families a remote region of Cappadocia, Turkey where a high incidence of the disease was noted. A “BAP1 cancer syndrome” was then described, which included uveal melanoma, clear cell renal carcinoma and cutaneous malignancies such as basal cell and squamous cell carcinomas (17,47). Recommendations for testing BAP1 mutations include patients diagnosed with mesothelioma under age 50 years or having multiple family members with mesothelioma or those associated with germline BAP1 mutations (47).
The clinical implications of BAP1 mutations or loss are still unclear. While germline BAP1 mutations are associated with development of malignancy, loss of BAP1 may actually correlate to improved prognosis (48). A SEER study from 1973 to 2010 compared the survival of 23 patients with BAP1 mutations to a control group of all patients with malignant mesothelioma (n=10,556) (46). Ten of the patients with genetic alterations had MPM, 10 had pleural mesothelioma and three had bicavitary disease. Actuarial median OS for BAP1 mutations was 5 years, vs. less than 1 year for control group. Five-year survival was 47% (95% CI, 24–67%) compared to 6.7% (95% CI, 6.2–7.3%) in the control group. The authors concluded that patients with germline BAP1 mutations have a 7-fold increase in long-term survival, which was independent of sex and age. These same conclusions were reached by a French National Network study from 2017, in which the status of BAP1 mutations were evaluated in peritoneal tissues from 46 MPM patients (49). Loss of BAP1 was only observed in epithelioid subtype tumors, which compromised 85% of the cohort. One inactivated BAP1 allele was seen in 73% of patients with MPM, though only 57% had complete loss of BAP1 protein expression. Patients with BAP1 mutations and BAP1 protein loss demonstrated better OS (P=0.004 and P=0.016 respectively). BAP1 loss in MPM may also be associated with a more inflammatory tumor microenvironment, which has sparked interest in potential applications of immune checkpoint inhibitors as therapy for patients with this genetic alteration (50,51).
Programmed death ligand 1 (PD-L1) is increasingly investigated in various malignancies, and has recently been shown to be clinically relevant in MPM. Cancer cells expressing PD-L1 enable tumor growth through CD4 and CD8 T-cell inactivation or T-cell apoptosis (52). Relationship of PD-L1 to mesothelioma has been explored, specifically to investigate the interplay of chronic inflammation caused by asbestos fiber with the immune system (53). Anti-PD-1 immunotherapy trials (either as monotherapy or in combination with anti-CTLA-4 agents) have thus far excluded patients with a primary MPM (50). A large prospective multicenter 2019 study assessed the prognostic role of PD-L1 in tumor samples from the phase III Bio-MAPS cohort randomized 448 patients with unresectable MPM with either pemetrexed plus cisplatin (n=223, 50%), vs. pemetrexed plus cisplatin plus bevacizumab (54). Tissue blocks were available to stain for PD-L1 in 212 patients. PD-L1 was expressed in 35.9% of samples (n=77). PD-L1 expression was significantly higher in patients with sarcomatoid and biphasic than epithelioid histology (P<0.001). In epithelioid patients (n=179), those with high PD-L1 staining (defined by the authors as >50% cells) had a longer median PFS (9.9 months) compared to 6.7 months (P=0.0047) in patients with low PD-L1 expression (<50% cells). Exposing the complex relationship between genetic and immune factors will provide further potential for precision treatment. For example, a 2019 study demonstrated higher expression of PD-L1 in BAP1-altered MPM (50). Immune checkpoint inhibitors, such as nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4) warrant further investigation in MPM.
Mesothelin is a cell surface glycoprotein expressed in mesothelial and peritoneal cells (43). It is also described as a tumor-associated antigen with high expression in pleural mesothelioma tumors. Three agents are currently being investigated as potential targets: SS1-P, a recombinant immunotoxin targeting mesothelin, MORAb-009, a chimeric monoclonal IgG1 antibody which blocks binding of mesothelin to CA-125, and CRS-207, a live-attenuated Listeria monocytogenes expressing human mesothelin (43). A 2019 open-label phase 1b study trialed injection of CRS-207 in combination with pemetrexed and cisplatin in 35 patients with unresectable pleural mesothelioma (55). Median PFS and OS was 7.5 (95% CI, 7.0–9.9) and 14.7 (95% CI, 11.2–21.9) months, respectively. Radiologic tumor size reduction was observed in 19 patients (31%). IHC pre- and post-injection of CRS-207 revealed increased infiltration of dendritic and natural killer (NK) cells, and possible proliferation of T-cells. The potential for mesothelin to serve as a therapeutic target is being examined via cancer stem cell models for MPM. Methods to test therapies for individual patient tumors in vitro are also being developed (56). Transglutaminase 2 (TG2) a GTP-binding regulatory protein, was found in a 2018 study by Adhikary et al. to play an important role in mesothelioma cancer stem cell survival (57). TG2 is highly expressed in mesothelioma tumor and stem cells. Knockdown of TG2 or treatment with TG2 inhibitors reduced in vitro stem cell spheroid formation, invasion, migration and tumor formation. TG2 specific inhibitors such as NC9 may serve as a future therapy target.
Additional immune and genetic targets for MPM include pulsed dendritic cells, for which a phase II open-label clinical trial is currently underway (58), and the phosphoinositide 3-kinase (PI3K) and MAPK pathways. A study employing dual color FISH analysis in 86 patients undergoing CRS-HIPEC for MPM noted that patients with combined homozygous deletions in CDKN2A and hemizygous NF2 loss negatively affected both PFS and OS (48). Patients in this category had similar survival rates to MPM patients before the advent of CRS-HIPEC, bringing into question whether this subtype of genetic alterations benefit from the current, standard treatment.
Conclusions
MPM is a rare and lethal disease of the peritoneal lining, with high variability in biologic aggressiveness. The standard of care for resectable disease remains CRS-HIPEC, with potential survival outcomes greater than 5 years in carefully selected patients. Patients with inoperable MPM can be offered systemic treatment, though the disease is largely refractory to standard chemotherapic regimens. Patients with MPM should be treated at high volume centers, with strong consideration for inclusion in tumor registries and clinical trials. In 2020, research will continue to explore promising genetic and immunologic targets, and focus on refinement of surgical methods to optimize CRS-HIPEC approaches.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Li CY, Alexander HR Jr. Peritoneal metastases from malignant mesothelioma. Surg Oncol Clin N Am 2018;27:539-49. [Crossref] [PubMed]
- Alexander HR Jr, Burk AP. Diagnosis and management of patients with malignant peritoneal mesothelioma. J Gastrointest Oncol 2016;7:79-86. [PubMed]
- Sugarbaker PH. Update on the management of malignant peritoneal mesothelioma. Transl Lung Cancer Res 2018;7:599-608. [Crossref] [PubMed]
- Enomoto LM, Shen P, Levine EA, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal mesothelioma: patient selection and special considerations. Cancer Manag Res 2019;11:4231-41. [Crossref] [PubMed]
- Miura JT, Johnston FM, Gamblin TC, et al. Current trends in the management of malignant peritoneal mesothelioma. Ann Surg Oncol 2014;21:3947-53. [Crossref] [PubMed]
- Turner KM, Varghese S, Alexander HR Jr. Surgery for peritoneal mesothelioma. Curr Treat Options Oncol 2011;12:189-200. [Crossref] [PubMed]
- Turaga KK, Deraco M, Alexander HR Jr. Current management strategies for peritoneal mesothelioma. Int J Hyperthermia 2017;33:579-81. [Crossref] [PubMed]
- Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686-93. [Crossref] [PubMed]
- Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol 2007;14:128-33. [Crossref] [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Cytoreductive surgery with selective versus complete parietal peritonectomy followed by hyperthermic intraperitoneal chemotherapy in patients with diffuse malignant peritoneal mesothelioma: a controlled study. Ann Surg Oncol 2012;19:1416-24. [Crossref] [PubMed]
- Ariche A, Klein Y, Cohen A, et al. Major hepatectomy for complex liver trauma. Hepatobiliary Surg Nutr 2015;4:299-302. [PubMed]
- Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis and pathogenesis. Curr Treat Options Oncol 2008;9:147-57. [Crossref] [PubMed]
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005 U.S. Mesothelioma Incidence Time Trend and Projections B. Price and A. Ware. Crit Rev Toxicol 2009;39:576-88. [Crossref] [PubMed]
- Yokota J. Tumor progression and metastasis. Carcinogenesis 2000;21:497-503. [Crossref] [PubMed]
- Broeckx G, Pauwels P. Malignant peritoneal mesothelioma: a review. Transl Lung Cancer Res 2018;7:537-42. [Crossref] [PubMed]
- Boussios S, Moschetta M, Karathanasi A, et al. Malignant peritoneal mesothelioma: clinical aspects, and therapeutic perspectives. Ann Gastroenterol 2018;31:659-69. [PubMed]
- Carbone M, Adusumilli PS, Alexander HR Jr, et al. Mesothelioma: scientific clues for prevention, diagnosis, and therapy. CA Cancer J Clin 2019;69:402-29. [Crossref] [PubMed]
- Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol 2007;18:985-90. [Crossref] [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J Clin Oncol 2009;27:6237-42. [Crossref] [PubMed]
- Alexander HR Jr, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779-86. [Crossref] [PubMed]
- Lee M, Alexander HR Jr, Burke A. Diffuse mesothelioma of the peritoneum: a pathological study of 64 tumours treated with cytoreductive therapy. Pathology 2013;45:464-73. [Crossref] [PubMed]
- Kaya H, Sezgi C, Tanrikulu AC, et al. Prognostic factors influencing survival in 35 patients with malignant peritoneal mesothelioma. Neoplasma 2014;61:433-8. [Crossref] [PubMed]
- Sugarbaker PH, Turaga KK, Alexander HR Jr, et al. Management of malignant peritoneal mesothelioma using cytoreductive surgery and perioperative chemotherapy. J Oncol Pract 2016;12:928-35. [Crossref] [PubMed]
- Mohr AM, Lavery RF, Barone A, et al. Angiographic embolization for liver injuries: low mortality, high morbidity. J Trauma 2003;55:1077-81; discussion 1081-2. [Crossref] [PubMed]
- Alexander HR Jr, Li CY, Kennedy TJ. Current management and future opportunities for peritoneal metastases: peritoneal mesothelioma. Ann Surg Oncol 2018;25:2159-64. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. In: Sugarbaker PH. editor. Peritoneal carcinomatosis: principles of management. Boston: Springer Science & Business Media, 1996.
- Yan TD, Deraco M, Elias D, et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database. Cancer 2011;117:1855-63. [Crossref] [PubMed]
- Llanos MD, Sugarbaker PH. Symptoms, signs and radiologic findings in patients having reoperative surgery for malignant peritoneal mesothelioma. Eur J Surg Oncol 2017;43:138-43. [Crossref] [PubMed]
- Lemoine L, Thijssen E, Carleer R, et al. Body surface area-based vs concentration-based perioperative intraperitoneal chemotherapy after optimal cytoreductive surgery in colorectal peritoneal surface malignancy treatment: COBOX trial. J Surg Oncol 2019;119:999-1010. [Crossref] [PubMed]
- Li YC, Khashab T, Terhune J, et al. Preoperative thrombocytosis predicts shortened survival in patients with malignant peritoneal mesothelioma undergoing operative cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2017;24:2259-65. [Crossref] [PubMed]
- Zaidi MY, Lee RM, Gamboa AC, et al. Preoperative risk score for predicting incomplete cytoreduction: a 12-institution study from the US HIPEC collaborative. Ann Surg Oncol 2020;27:156-64. [Crossref] [PubMed]
- Witkamp AJ, De Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 2001;27:365-74. [Crossref] [PubMed]
- Kusamura S, Torres Mesa PA, Cabras A, et al. The role of Ki-67 and pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DMPM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2016;23:1468-73. [Crossref] [PubMed]
- Votanopoulos KI, Sugarbaker P, Deraco M, et al. Is cytoreductive surgery with hyperthermic intraperitoneal chemotherapy justified for biphasic variants of peritoneal mesothelioma? Outcomes from the Peritoneal Surface Oncology Group International Registry. Ann Surg Oncol 2018;25:667-73. [Crossref] [PubMed]
- Van der Speeten K, Lemoine L, Sugarbaker P. Overview of the optimal perioperative intraperitoneal chemotherapy regimens used in current clinical practice. Pleura Peritoneum 2017;2:63-72. [Crossref] [PubMed]
- Malgras B, Gayat E, Aoun O, et al. Impact of combination chemotherapy in peritoneal mesothelioma hyperthermic intraperitoneal chemotherapy (HIPEC): the RENAPE study. Ann Surg Oncol 2018;25:3271-9. [Crossref] [PubMed]
- Blackham AU, Shen P, Stewart JH, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: Mitomycin versus cisplatin. Ann Surg Oncol 2010;17:2720-7. [Crossref] [PubMed]
- Foster JM, Sleightholm R, Patel A, et al. Morbidity and mortality rates following cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy compared with other high-risk surgical oncology procedures. JAMA Netw Open 2019;2:e186847. [Crossref] [PubMed]
- Badrudin D, Sideris L, Perrault-Mercier C, et al. Comparison of open and closed abdomen techniques for the delivery of intraperitoneal pemetrexed using a murine model. J Surg Oncol 2018;117:1318-22. [Crossref] [PubMed]
- Kusamura S, Azmi N, Fumagalli L, et al. Phase II randomized study on tissue distribution and pharmacokinetics of cisplatin according to different levels of intra-abdominal pressure (IAP) during HIPEC (NCT02949791). Eur J Surg Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Goldenshluger M, Zippel D, Ben-Yaacov A, et al. Core body temperature but not intraabdominal pressure predicts postoperative complications following closed-system hyperthermic intraperitoneal chemotherapy (HIPEC) administration. Ann Surg Oncol 2018;25:660-6. [Crossref] [PubMed]
- McConnell YJ, Mac K LA, Francis WP, et al. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol 2013;107:591-6. [Crossref] [PubMed]
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211-8. [Crossref] [PubMed]
- Simon GR, Verschraegen CF, Jänne PA, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol 2008;26:3567-72. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Baumann F, Flores E, Napolitano A, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis 2015;36:76-81. [Crossref] [PubMed]
- Kittaneh M, Berkelhammer C. Detecting germline BAP1 mutations in patients with peritoneal mesothelioma: benefits to patient and family members. J Transl Med 2018;16:194. [Crossref] [PubMed]
- Singhi AD, Krasinskas AM, Choudry HA, et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol 2016;29:14-24. [Crossref] [PubMed]
- Leblay N, Leprêtre F, Le Stang N, et al. BAP1 is altered by copy number loss, mutation, and/or loss of protein expression in more than 70% of malignant peritoneal mesotheliomas. J Thorac Oncol 2017;12:724-33. [Crossref] [PubMed]
- Ladanyi M, Sanchez Vega F, Zauderer M. Loss of BAP1 as a candidate predictive biomarker for immunotherapy of mesothelioma. Genome Med 2019;11:18. [Crossref] [PubMed]
- Shrestha R, Nabavi N, Lin YY, et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med 2019;11:8. [Crossref] [PubMed]
- Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017;8:2171-86. [PubMed]
- Cornelissen R, Heuvers ME, Maat AP, et al. New roads open up for implementing immunotherapy in mesothelioma. Clin Dev Immunol 2012;2012:927240. [Crossref] [PubMed]
- Brosseau S, Danel C, Scherpereel A, et al. Shorter survival in malignant pleural mesothelioma patients with high PD-L1 expression associated with sarcomatoid or biphasic histology subtype: a series of 214 cases from the bio-MAPS cohort. Clin Lung Cancer 2019;20:e564-75. [Crossref] [PubMed]
- Hassan R, Alley E, Kindler H, et al. Clinical response of live-attenuated, Listeria monocytogenes expressing mesothelin (CRS-207) with chemotherapy in patients with malignant pleural mesothelioma. Clin Cancer Res 2019;25:5787-98. [Crossref] [PubMed]
- Varghese S, Whipple R, Martin SS, et al. Multipotent cancer stem cells derived from human malignant peritoneal mesothelioma promote tumorigenesis. PLoS One 2012;7:e52825. [Crossref] [PubMed]
- Adhikary G, Grun D, Alexander HR Jr, et al. Transglutaminase is a mesothelioma cancer stem cell survival protein that is required for tumor formation. Oncotarget 2018;9:34495-505. [Crossref] [PubMed]
- de Boer NL, van Kooten JP, Burger JWA, et al. Adjuvant dendritic cell based immunotherapy (DCBI) after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal mesothelioma, a phase II single centre open-label clinical trial: rationale and design of the MESOPEC trial. BMJ Open 2019;9:e026779. [Crossref] [PubMed]


