Neoadjuvant and adjuvant epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) therapy for lung cancer
Almost 1.3 million new cases of non-small lung cancer (NSCLC) every year are diagnosed throughout the whole world (1). Approximately one out of every five these patients are diagnosed with locally advanced disease and have no opportunities of surgical resection. Even though perioperative sequential or concurrent chemoradiotherapy (CRT) are standard treatment and part of patients could benefit moderately on respect of disease-free survival (DFS), overall prognosis of locally advanced patients is still quite poor. Oncologists began to pay more attention to perioperative targeting therapy. Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) have shown excellent efficacy in advanced EGFR-mutation positive NSCLC, with almost 70% of response rate. Several randomized controlled trials (RCTs) have confirmed that first-line EGFR-TKI regimen can improve tumor response rate and progression-free survival (PFS) in patients with advanced EGFR-mutant NSCLC (2-4). But it still remains appealing to explore whether EGFR-TKI can be applied to neoadjuvant/adjuvant therapy for resectable stages (5), in order to improve DFS and overall survival (OS). Upon EGFR-TKIs appearing on the market, some surgeons began recommending TKIs during perioperative period. However, since applications of TKI perioperatively are associated with novel concepts in translational researches, it still remains a highly controversial issue up to now. Different biological characteristics between early and advanced lung cancer can be detected in previous reports and proved by several phenomena. For example, high levels of serum tumor markers, such as carcinoembryonic antigen (CEA), cytokeratin19 fragment antigen 21-1 (CYFRA21-1) and cancer antigen 125 (CA125), are negative prognostic factors in early-stage NSCLC but positive prognostic factors in advanced NSCLC (6); early stage tumors are more mobile than locally advanced stage NSCLC, which need to be integrated into precise radiotherapy planning (7). Therefore, first-line regimens target continuously at multiple heterogeneous targets until disease progression, while adjuvant and neoadjuvant treatments in early lung cancers mainly act on relatively homogeneous targets and thus achieve higher response rate. By contrast, TKI adjuvant therapy is expected to eliminate potential circulating tumor cells (CTCs), instead of substantial lesions. Then there come to several problems to be clarified. For example, whether discontinuation of TKI before resistance occurs would change tumors’ biological behaviors and lead to resistance when relapse; whether a rebound effect or disease flare occurs earlier than average since oncogenes get rid of additive inhibition effect after TKI neoadjuvant treatment is completed. So in this article, we will discuss applications of adjuvant and neoadjuvant EGFR-TKI therapies in NSCLC.
Adjuvant EGFR-TKI therapy
Adjuvant therapy acts mostly by clearing away a small amount of CTCs or residual tumor cells, thus increasing cure rate of lung cancers. CTCs are malignant cells originating from primary tumor mass and formed by invading vessels to spread to distant sites or organs. So TKIs, targeting at cells with EGFR mutations, will eliminate CTCs due to similar genotype and molecular expression to malignant cells in primary tumor site. As to patients who have a larger quantity of residual cancer cells or are insensitive to regular treatment, prolonging DFS may be the second best achievement. That means applying first-line regimens initially for advanced cancer to relatively early stage lung cancer patients before tumor lesions become evident in imaging.
The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis and its RCTs have validated the importance of platinum-based doublet chemotherapy as adjuvant therapy in NSCLC patients, with 5.3% advantage of 5-year survival rate in chemotherapy group over the control group (48.8% and 43.5%, respectively) (8). Besides, successful adjuvant targeting therapies have been reported in fields of other cancers, such as imatinib for the treatment of gastrointestinal stromal tumors (GIST) and herceptin for breast cancer. Despite these triumphs, failures predominate. Avastin, used as postoperative therapy for colon cancer, was evaluated in the recent National Surgical Adjuvant Breast and Bowel Project (NSABP) C-08 study (9). The survival curves separated to some extent, but converged soon after drug discontinuation and became indistinguishable at the point of 3.5 years, suggesting no benefits in 3-year DFS or long-term cure rate in stage II/III colon cancer patients. This study indicated that colon cancer was inhibited from recurrence during the course of avastin, but rebounded again accompanied with withdrawal. Researchers hypothesized that residual cancer cells were merely inhibited transiently and that early and advanced cancers might exhibit different biological behaviors. Analogically, we could also take early and advanced NSCLCs separately into consideration when evaluating efficacy of erlotinib or gefitinib. Subsequently, many clinical trials on TKIs have been conducted to figure out this puzzle. The phase III NCIC CTG BR19 study was based on general population and recruited patients more than 18 years old with completely resected and pathologically confirmed stage IB/II/IIIA NSCLC (51.69%, 34.79%, 13.32%, respectively) (10). Five hundred and three patients were enrolled and randomized to gefitinib group or placebo group (251:252, respectively). The primary endpoint is OS and secondary endpoints included DFS, toxicity and establishment of a tumor bank for biomarker analysis. With a median duration of 4.8 and 8.9 months (gefitinib and placebo group, respectively) for treatment, no significant benefits or differences on OS and DFS were detected either in two groups or in subgroups (based on mutation status of EGFR or KRAS), even worse in the EGFR subgroup. We analyze BR19 study retrospectively and explanations could be as follows: (I) the median duration of treatment was different between treatment arms and shorter than usual randomized, multicenter, double-blind clinical trials. So the poor results may result more from disease progressions rather than the inefficacy of TKIs; (II) a low rate of EGFR mutation (only 15 positive tumors, 3.0%, 15/503) may act as interference factors. Why there exists such a low mutation rate? Firstly, it is because of the non-selected population. As far as we’re concerned, EGFR mutation rate in Asian, women and non-smokers are relatively higher, but rates of patients in BR19 study are just 46.12% of Asian, 1.79% of women and 8.35% of non-smokers. Secondly, there might be actually a lower mutation rate in early NSCLC than advanced NSCLC (in this study, stage IB/II, 51.69%:34.79% respectively, and 86.48% totally), indicating that early and advanced NSCLCs may represent different biologic characteristics, or that prophase treatment (chemotherapy, radiotherapy or CRT) has transformed biological behaviors of tumors. Further studies are warranted to confirm these hypotheses; (III) EGFR pathway might not function as the primary role in stage I/II NSCLC. Nevertheless, BR19 did demonstrated a meaningful prognostic effect on OS [hazard ratio (HR): 0.57; 95% confidence interval (CI): 0.14-2.33] and noticeable tolerability to TKIs, inspiring more further trials on TKI adjuvant therapy.
Overall, these trials indicate that appropriate selection of patients with EGFR mutations is quite necessary to avoid misleading conclusions. Therefore, a retrospective study (11) performed at the Memorial Sloan Kettering Cancer Center (MSKCC) recruited 167 completely resected lung adenocarcinoma patients harboring EGFR mutations (stage IB 70%, II 15%, III 15%) after standard adjuvant chemotherapy and radiotherapy. Results suggested that TKI adjuvant therapy (gefitinib or erlotinib) had a tendency to improve 2-year DFS rate compared with platinum-based chemotherapy alone (89% vs. 72%, respectively; HR: 0.53, 95% CI: 0.28-1.03, P=0.06). However, with a TKI median treatment time of 20 months, DFS curves separated in the middle and converged at two ends, similar results as shown in C-08 study of colon cancer. It can be extrapolated that adjuvant TKI regimens lead to acquired resistance to EGFR-TKIs. According to follow-ups, patients with disease progression after TKI adjuvant therapy retained sensibility (73%) to TKI retreatment and a 10-month median PFS, close to that in first-line TKI therapy for advanced NSCLC. This study in MSKCC is the first clinical trial to demonstrate that EGFR mutant NSCLC patients after R0 surgery can benefit from erlotinib or gefitinib as adjuvant therapy. However, although their cohort targeted at selected population with EGFR mutations and receiving uniform testing and treatment in the same institute, there still exist several limitations. That is to say, researchers should lay more emphasis on how to rule out oncologists’ preferences, prolong the primary endpoint to 5-year DFS rather than 2-year DFS, and eliminate the quantity imbalance between arms.
Subsequently, how should we monitor tumor behaviors and evaluate effects of adjuvant TKI therapy in order to change or moderate regimens timely once disease relapse? Pathological evidences from re-biopsies are universally acknowledged as gold standards and treatment plans are regulated according to different levels of complete response, partial response or progressed disease (12). However, multiple biopsies during following management of postoperative patients are expensive, exhausting and unnecessary, and thus detecting CTC in plasma would attract more attention of oncologists and patients (13-15). CTC can be considered as “fluid biopsy”, monitoring EGFR mutation status and identifying resistance to treatment and distant metastasis as early as possible. This not only assists to regulate TKI regimens and make clinical decisions, but also acts as an effective prognosis factor. Unfortunately, CTCs exist in extreme rarity in circulating blood and present techniques are not that sensitive enough to identify minor changes of CTC in peripheral blood. More advanced techniques are imperative to be applied.
Therefore, it comes to us another puzzle accompanying these trials that what should we do to identify therapeutic strategies when encountering with inevitable drug resistance. Genetic analyses of drug resistance (16) showed that there was a significantly higher frequency of T790M mutation among patients who developed disease progression during TKI continuation than those after discontinuation. It was also observed that certain genetic mechanisms of acquired resistance disappeared in the absence of TKI, and that a second round of TKI treatment could be effective. Preclinical experiments have indicated that T790M can be induced by a low level of TKI concentration and diminish during TKI holiday. As to patients with acquired resistance, TKI withdrawal from cell lines will lead to a gradually increasing loss of T790M mutation, and subsequently T790M cells are getting relative more indolence and deceleration compared with EGFR-sensitive clones (17). All of these imply a promising future of TKI retreatment and offer further support for TKI adjuvant and neoadjuvant therapy (16,18).
EGFR-TKI neoadjuvant therapy
Preoperative chemotherapy theoretically has the potential to make tumor mass shrink, improve complete resection rate, eliminate micrometastases and reduce risks of recurrence. Although neoadjuvant therapy might delay surgeries and take risks of disease progression, its advantages have occupied solid foundation. Kinds of RCTs reported neoadjuvant chemotherapy could improve survival, although preoperative treatment varied from chemotherapy with or without radiotherapy and apparent imbalance between groups resulted in bias. Several researches were conducted (19-29) mainly comparing preoperative chemotherapy with surgery alone, most of which stopped ahead of schedule due to poor accrual or high disease progression rate. The role of preoperative chemotherapy is finally getting rid of controversy when NSCLC Meta-analysis Collaborative Group (30) validate its effect for patients with resectable NSCLC. This meta-analysis includes 15 RCTs and is based on individual patient data (2,385 patients). Results demonstrated that neoadjuvant chemotherapy could absolutely improve 5-year OS, from 40% to 45%.
Along with the arrival of era of molecular-oriented treatment, especially benefits from postoperative TKI administration, oncologists began to seek strong evidences to apply TKI from advanced to early EGFR mutation positive NSCLC. Since 2007, several case reports involving patients with stage IIIA-N2 suggested that TKI neoadjuvant therapy could induce tumor downstaging. EGFR mutant cancer cells went through shrinkage and even obliteration compared with the original number of malignant cells detected in circulating blood, improving complete resection rate. Unfortunately, no follow-up survival data was reported, thus failing to provide stronger evidences (31-34).
The first prospective study conducted by University of Toronto (35) recruited 36 patients with biopsy-confirmed clinical stage I NSCLC, who received complete resection after undertaking preoperative gefitinib treatment for 28 days. In the process of exploring how histopathological types affect biologic characteristics of tumors, researchers figured out that there is a higher radiological response rate to gefitinib treatment in tumors with reduced tumor cellularity and proliferative index detected in EGFR mutation positive adenocarcinoma. Then the consequent prospective, open-label, single-arm, phase II study (n=36) (36) regarding to neoadjuvant TKIs was conducted at the same institute. Treated with gefitinib 250 mg/day as neoadjuvant therapy for 28 days, 8.3% (3/36) patients developed tumor progression, and overall response rate was only 11%. Response rate among patients harboring EGFR mutations (17% of total patients) was merely 50%, which was not as favorable as expected. In spite of poor results, researchers demonstrated neoadjuvant TKI therapy is feasible and safe, and would not affect healing of wounds or bronchial stumps. Subsequently, pathological features of specimens from patients undergoing surgeries after TKI neoadjuvant therapy, showed that typical structural changes varied accordingly, such as reduction of tumor cellularity, deceleration in tumor cell proliferation index, a noticeable replacement of tumors by fibrotic scar tissue, and concentration of focal residual tumors limited in areas of fibrous stroma and lymphocyte infiltration (35,36). Histopathological types are associated with clinical response rate and tumor structures transformed under the pressure of TKI inhibition, which appeals for promising clinical trials to explore impacts of TKIs and other novel targeting treatment in NSCLC.
In 2010, a retrospective analysis conducted by Japan Clinical Oncology Group via a questionnaire survey (37), reported that nine patients with advanced (stage IIIA-IV, inoperable) EGFR mutation positive adenocarcinoma underwent salvage surgeries if tumor downstaging to stage I/II and serum tumor markers recovering to normal levels were observed after preoperative gefitinib treatment. Although PFS was only 6 months and OS was up to 32 months, induced TKI therapy with sequential surgeries promises a bright future.
In 2012, the department of Surgical Oncology in Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital conducted a phase II, open-label study of neoadjuvant tarceva for early-stage resectable NSCLCs (38). Fifteen selected patients from an enriched population (never-smoker, female, Asian ethnicity and non-squamous carcinoma) and 60 unselected patients were enrolled. Patients took preoperative erlotinib 150 mg/day for 3 weeks, and primary endpoints included pathologic response rate and toxicity based on positron emission tomography/computed tomography (PET/CT) scan. PET/CT scan showed a metabolic partial response in 16 patients (enriched, n=10; unselected, n=6). CT scan according to response evaluation criteria in solid tumors (RECIST) showed a radiologic partial response in three patients (4.0%, 3/75, all in the enriched population), and 14 patients (18.7%, 14/75) had significant pathologic response (enriched, n=11; unselected, n=3). This study suggested that neoadjuvant erlotinib has more potential efficacy and fewer adverse effects, especially among enriched population.
Several case reports (39-41) and clinical trials with small sample size have validated advantages of preoperative TKI monotherapy. Retrospective analyses (Table 1) are appealing for further prospective researches of TKI neoadjuvant therapy in stage IIIA-N2 NSCLC. In January 2008, Guangdong Lung Cancer Institute registered a factorial assignment, single center, non-randomized, open label, phase II study to assess efficacy of TKI and TKI related toxicity, with a setting of control group recruiting EGFR wild-type NSCLC. Stage IIIA-N2 was confirmed by mediastinoscopy or PET/CT. EGFR mutant patients in experimental group took erlotinib with a median treatment time prolonged from 30 to 42 days, while EGFR wild-type NSCLCs underwent three cycles of neoadjuvant gemcitabine-carboplatin therapy. Efficacy assessment was conducted at the end of 42 days in erlotinib group and three cycles of chemotherapy in control group. Patients rendered free of disease progression received radical surgeries and those whose disease progression received second-line chemotherapy or concurrent CRT. Primary end point was objective response rate (ORR) and secondary end points included tumor downstaging, complete resection rate, side effects, PFS and OS. Simon’s two-stage design for phase II studies was used to calculate optimal sample size. Up to now, enrollment in first period has been completed with 12 patients in each group. Only six patients in erlotinib group received surgeries (R0:R1 =3:3) and seven in control group (R0:R1 =5:2). Although response rate in erlotinib group with EGFR mutant-type was higher than that in control group with EGFR wild-type, no significant differences were observed between two groups in respect of ORR, clinical downstaging, pathological response rate or complete resection rate. Disease failure models in gemcitabine/cisplatin (GC) group were local recurrence and distant metastasis (4:5, respectively); while in erlotinib group, nine patients developed distant metastasis, up to 81.82%, and three of them suffered from brain metastasis. One of explanations might be rebound effect or disease flare after TKI withdrawal in advanced NSCLC patients reported by Riely and colleagues (42). After discontinuation of TKI, residual CTCs have accelerated proliferation and rebound effect, resulting from oncogene-driven malignancies suddenly relieved from inhibitions. Besides, no significant differences about PFS and OS were figured out between two groups. Although patients in GC group benefited more with regard to DFS, those in erlotinib group seemed to have a longer OS. It was observed that four patients in erlotinib group responded well to second round of TKI even when disease progressed after surgeries. PFS after disease progression in experimental group was longer than that in control group (8 and 4 months, respectively), which might due to reinstitution of TKI and have also explained why erlotinib tends to improve OS. Despite that there existed bias produced by different biological features of EGFR mutant and wild type between two groups, results and conclusions in this study are of crucial value of providing basic materials and proof to validate efficacy of TKI neoadjuvant therapy.
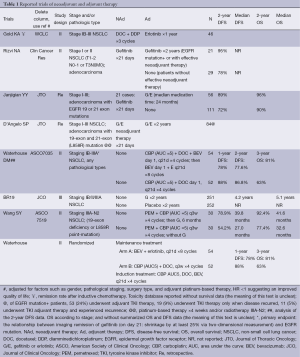
Full table
Ongoing trials on perioperative EGFR-TKI therapy
Sequist and colleagues at MSKCC launched an ongoing single-arm, multicenter phase II clinical trial of adjuvant erlotinib in surgically resected early-stage EGFR mutation-positive NSCLC (SELECT). Eligible criteria include resected stage IA-IIIA NSCLC patients harboring TKI-sensitizing EGFR mutations and having completed standard adjuvant chemotherapy and/or radiotherapy. Patients took oral erlotinib 150 mg/day continuously for 2 years. The primary endpoint was 2-year DFS and secondary endpoints are safety, tolerability and OS. Preliminary results about a sample size of 100 patients were reported on American Society of Clinical Oncology (ASCO) both in 2012 and 2014. Although median DFS has not be reached, 2-year DFS was longer than historical data (89% vs. 76%, respectively) with median follow-up of 3.4 years among patients with complete resection. However, it was observed that 25 patients developed recurrence in average 12 months after TKI withdrawal and only four during TKI administration. Those 29 patients received re-biopsies and pathological results showed there were fourteen recurrence originating from primary activated mutations, one acquired T790M during TKI treatment and three unable to detect or diagnose with confirmation. Regardless of recurrence, patients remain sensitive to second round of erlotinib and tolerable to median treatment time of 10 months. Unfortunately, some patients had to reduce drug dose or even withdraw TKI administration due to toxicities of erlotinib. Researchers concluded that erlotinib adjuvant therapy could inhibit tumor cell proliferation and reduce micrometastatic lesions. However, it should be noticed that there was a huge proportion of relatively early NSCLC (stage I/II/III, 45% vs. 27% vs. 28%, respectively) and that the DFS curve about stage II patients was even lower than that about stage III patients, indicating that relatively early stage NSCLCs might benefit less from TKI neoadjuvant therapy. In addition, this single-arm, phase II study is lack of long-term follow-up results drawn from RCTs, thus having difficulty in confirming efficacy of postoperative TKI regimens. Nevertheless, SELECT provides hints that TKIs might merely block EGFR-tyrosine kinase pathway transiently and delay disease progression, and that oncogene-driven malignancies with EGFR mutations obtain sensitivity to TKI again after “TKI holiday”. Therefore, it is imperative to design trials exploring efficacy with TKI administration longer than 2 years. Final results of SELECT are quite worthy of expect.
Other ongoing RCTs about TKI adjuvant therapy, such as RADIANT and TASTE, are both based on EGFR IHC/FISH-positive population (Table 2). In ASCO, 2014, RADIANT (randomized, double-blind, phase III clinical trial) was reported, aiming to evaluate efficacy of erlotinib adjuvant therapy vs. placebo therapy in completely resected NSCLC patients harboring high EGFR gene copy numbers or overexpression of EGFR protein. Patients were randomly assigned 2:1 to erlotinib group and placebo group. Significantly more patients developed brain relapse in erlotinib group than in placebo group (40% vs. 12.9%, respectively). The 2-year DFS rate (0.75 vs. 0.54) and 4-year DFS rate (0.43 vs. 0.43) in both groups, indicated that TKIs just delay tumor recurrence rather than eliminate chances of recurrence. Subgroup analyses indicate that DFS in erlotinib group was better than that in placebo group with regard to patients with Del 19exon and 21exon mutation (L858R). Although results show that patients in experimental group suffer from higher risk of recurrence than in placebo group by almost 40%, there exist certain defects and limitations of data interpretation. Firstly, EGFR FISH (+) DFS was considered as primary endpoint during 2009 and 2010, but was deleted in revised evaluation criteria in December, 2010. Accordingly, DFS and OS in EGFR mutation subgroup were added as secondary endpoints. Secondly, certain significant bias and limitations exist due to the imbalance of stage within groups, different usage of adjuvant chemotherapy and tumor size. Last but not least, overall treatment time of TKI varied from 2 to 22 months, and only less than 60% patients took oral TKI continuously for over 1 year owing to intolerable toxicities or lack of treatment compliance. Therefore, neither the inefficacy of TKIs nor the short time of TKI treatment is to be blamed on poor DFS and OS. Further researches with high unity of treatment model and patients’ compliance are warranted.
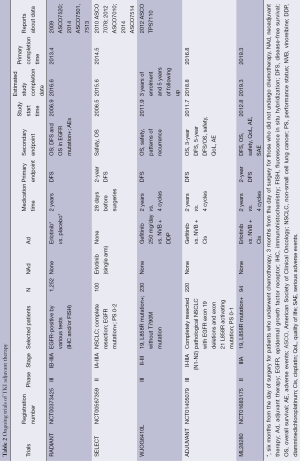
Full table
Studies like SELECT and RADIANT must recruit almost 900 cases to detect small differences between groups due to low EGFR mutation rates in European and American populations. So what about Asian ethnicity among which EGFR mutation rate is relatively high?
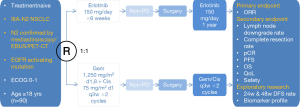
Results of clinical trials have suggested that early and advanced EGFR-mutant NSCLCs may have different disease profiles and different biological characteristics, and be responsive to TKI perioperative therapy based on different mechanisms. But large quantities of head-to-head clinical comparative trials are needed to provide more strong evidences. Selection criteria for TKI adjuvant therapy should select patients at high risk of recurrence, such as those with stage II-IIIA and lymph node metastasis, and EGFR mutation positive NSCLCs which is quite sensitive to TKIs. These two criteria are coordinated with an advantage that EGFR mutation rate in China is as up to 40-50%. By taking factors mentioned above into consideration and ruling out some bias, several ongoing clinical trials on TKI adjuvant therapy are emerging and showing up. In September 2011, two multi-center, randomized, open-level, phase III clinical trials were launched (CTONG1104/ADJUVANT and CTONG1103/EMERGING, Figures 1,2), both of which were designed to evaluate efficacy of perioperative TKI treatment among resectable EGFR mutation-positive NSCLC patients.
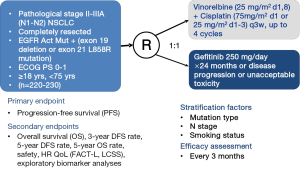
CTONG1104 (NCT01405079, Figure 1) is a national, multicenter, randomized, open-level, phase III trial of gefitinib vs. combination of vinorelbine/platinum as adjuvant treatment in pathological stage II-IIIA (N1-2) NSCLC with EGFR activating mutations. This clinical trial represents a success in transforming TKI administration from a subsetting of advanced NSCLC to a subsetting of relatively earlier NSCLC after complete resection. CTONG1104 recruited NSCLC patients with pathologically confirmed mediastinal lymph node metastasis (N1/N2 positive). Other main inclusion criteria are such like patients who are 18-75 years old, have undergone complete resection with pathological stage II-IIIA (N1-2) NSCLC with EGFR exon 19 deletions or 21 L858R activating mutations, and without any systemic function failures. Patients were randomly assigned into gefitinib group or chemotherapy group. Patients in experimental arm take gefitinib 250 mg/day orally continuously for 1 year or until disease progression or intolerable toxicity, while patients in control group receive vinorelbine 25 mg/m2 on days 1 and 8 plus cisplatin 75 mg/m2 on day 1 for four cycles, or just discontinue when disease progression or unacceptable toxicity. The primary endpoint is DFS and secondary endpoints are OS, 3-year and 5-year DFS rate, 5-year OS rate, adverse events and quality of life (QoL). According to agenda planned, 220 cases would have been enrolled and assigned into two groups within 2 years, have collected follow-up data within 5 years, and have drawn a final conclusion within 8 years. Researchers have learnt a lesson from previous trials and set up more rigorous criteria for including and excluding patients. Given the current acknowledge that adjuvant therapy targets at potential circulating cells, CTONG1104 provides a novel treatment to postoperative patients and assists to enlarge applications of TKIs and improve cure rate of NSCLCs.
CTONG1103 (NCT01407822, Figure 2) is a national, multicenter, randomized, open-level, phase II trial of erlotinib vs. combination of gemcitabine plus cisplatin as neoadjuvant treatment in stage IIIA-N2 NSCLC with sensitizing EGFR mutation in exon 19 or 21. Stage IIIA-N2 NSCLC stands for a relatively heterogenerous group of patients with ipsilateral mediastinal lymph node involvement and its response to treatment modalities is still suspended in the air. What’s worse, concurrent CRT as the standard treatment for stage IIIA-N2 NSCLC brings out drug-induced adverse effects, and clinical applications of CRT are subsequently limited. Based on all the advantages and defaults mentioned above this article, CTONG1103 was designed to recruit patients with stage IIIA-N2 EGFR activating mutation NSCLC confirmed preoperatively by PET/CT, endobronchial ultrasound (EBUS) or mediastinoscopy and evaluate efficacy and safety of erlotinib vs. GC as neoadjuvant therapy. Chief inclusion criteria include patients with EGFR activating mutation in exon 19 or 21 confirmed by biopsy of primary tumor or N2 lymph nodes and performance status (PS) 0-1. Besides, patients should not have prior exposure to any TKIs, chemotherapy or any other kind of systemic anti-tumor therapies. During perioperative period, patients were assigned randomly to erlotinib group or GC group. In erlotinib group, patients firstly take oral erlotinib for 6 weeks before operations; when evaluated as non-progressive disease (PD), patients continued erlotinib for 1 year after complete surgeries. In control group, two preoperative cycles of GC (gemcitabine 1,250 mg/m2 on days 1 and 8 plus cisplatin 75 mg/m2 on day 1 of a 3-week schedule); and as the same criteria as erlotinib group, two postoperative cycles of adjuvant chemotherapy were administered. The primary endpoint in both groups was the ORR of adjuvant therapies, and secondary endpoints are PFS, complete resection rate, pathological complete response, 3-year OS rate, adverse events and QoL. When faced with stage IIIA-N2 NSCLC which is marginalized between operable and inoperable, CTONG1103 is quite emerging just as it’s called EMERGING to explore a brand new treatment strategy for this subsetting.
Currently, the role of EGFR TKIs in perioperative early-stage NSCLC with EGFR activating mutations has not been established and validated. Therefore, both of these two trials take priorities on respect of providing high-level evidence and initiating the beginning of a new era. Only when further RCTs of neoadjuvant combined with adjuvant TKI therapy with huge sample size are brought forward, can researchers treat malignant cancers as chronic diseases with strong confidence. Stepping ahead on giants’ shoulders, we are looking forward to these two promising trials CTONG1103 and CTONG1104 to obtain more evidence to guide customized therapy in resectable NSCLC patients (Tables 1 and 2).
Acknowledgements
Funding: National Natural Science Foundation of China (81001031, 81372285); the Natural Science Foundation of Guangdong Province (S2013010016354).
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [PubMed]
- West H. The evolving role of targeted therapy in early-stage and locally advanced non-small cell lung cancer. Curr Oncol Rep 2011;13:280-9. [PubMed]
- Cedrés S, Nuñez I, Longo M, et al. Serum tumor markers CEA, CYFRA21-1, and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC). Clin Lung Cancer 2011;12:172-9. [PubMed]
- Yu ZH, Lin SH, Balter P, et al. A comparison of tumor motion characteristics between early stage and locally advanced stage lung cancers. Radiother Oncol 2012;104:33-8. [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [PubMed]
- Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011;29:11-6. [PubMed]
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [PubMed]
- Janjigian YY, Park BJ, Zakowski MF, et al. Impact on disease-free survival of adjuvant erlotinib or gefitinib in patients with resected lung adenocarcinomas that harbor EGFR mutations. J Thorac Oncol 2011;6:569-75. [PubMed]
- Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol 2014;15:e42-50. [PubMed]
- O'Flaherty JD, Gray S, Richard D, et al. Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer 2012;76:19-25. [PubMed]
- Costa DB, Kobayashi S, Tenen DG, et al. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer 2007;58:95-103. [PubMed]
- Wendel M, Bazhenova L, Boshuizen R, et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys Biol 2012;9:016005. [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [PubMed]
- Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011;17:1616-22. [PubMed]
- Oxnard GR, Janjigian YY, Arcila ME, et al. Maintained sensitivity to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer recurring after adjuvant erlotinib or gefitinib. Clin Cancer Res 2011;17:6322-8. [PubMed]
- Dautzenberg B, Benichou J, Allard P, et al. Failure of the perioperative PCV neoadjuvant polychemotherapy in resectable bronchogenic non-small cell carcinoma. Results from a randomized phase II trial. Cancer 1990;65:2435-41. [PubMed]
- Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst 1994;86:673-80. [PubMed]
- Rosell R, Gómez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. N Engl J Med 1994;330:153-8. [PubMed]
- Depierre A, Milleron B, Moro-Sibilot D, et al. Preoperative chemotherapy followed by surgery compared with primary surgery in resectable stage I (except T1N0), II, and IIIa non-small-cell lung cancer. J Clin Oncol 2002;20:247-53. [PubMed]
- Nagai K, Tsuchiya R, Mori T, et al. A randomized trial comparing induction chemotherapy followed by surgery with surgery alone for patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209). J Thorac Cardiovasc Surg 2003;125:254-60. [PubMed]
- Mattson KV, Abratt RP, ten Velde G, et al. Docetaxel as neoadjuvant therapy for radically treatable stage III non-small-cell lung cancer: a multinational randomised phase III study. Ann Oncol 2003;14:116-22. [PubMed]
- Waller D, Peake MD, Stephens RJ, et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg 2004;26:173-82. [PubMed]
- Gilligan D, Nicolson M, Smith I, et al. Preoperative chemotherapy in patients with resectable non-small cell lung cancer: results of the MRC LU22/NVALT 2/EORTC 08012 multicentre randomised trial and update of systematic review. Lancet 2007;369:1929-37. [PubMed]
- Pisters KM, Vallières E, Crowley JJ, et al. Surgery with or without preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer: Southwest Oncology Group Trial S9900, an intergroup, randomized, phase III trial. J Clin Oncol 2010;28:1843-9. [PubMed]
- Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized phase III study of surgery alone or surgery plus preoperative cisplatin and gemcitabine in stages IB to IIIA non-small-cell lung cancer. J Clin Oncol 2012;30:172-8. [PubMed]
- Felip E, Rosell R, Maestre JA, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol 2010;28:3138-45. [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [PubMed]
- Wang Q, Wang H, Li P, et al. Erlotinib-based perioperative adjuvant therapy for a case of unresectable stage IIIA (N2) nonsmall cell lung cancer. Am J Med Sci 2010;340:321-5. [PubMed]
- Kappers I, Klomp HM, Burgers JA, et al. Neoadjuvant (induction) erlotinib response in stage IIIA non-small-cell lung cancer. J Clin Oncol 2008;26:4205-7. [PubMed]
- Takamochi K, Suzuki K, Sugimura H, et al. Surgical resection after gefitinib treatment in patients with lung adenocarcinoma harboring epidermal growth factor receptor gene mutation. Lung Cancer 2007;58:149-55. [PubMed]
- Shen H, Zhong X, Ge XQ, et al. Surgical resection of lung adenocarcinoma without EGFR mutation after neoadjuvant gefitinib treatment. Clin Respir J 2010;4:192-3. [PubMed]
- Lara-Guerra H, Chung CT, Schwock J, et al. Histopathological and immunohistochemical features associated with clinical response to neoadjuvant gefitinib therapy in early stage non-small cell lung cancer. Lung Cancer 2012;76:235-41. [PubMed]
- Lara-Guerra H, Waddell TK, Salvarrey MA, et al. Phase II study of preoperative gefitinib in clinical stage I non-small-cell lung cancer. J Clin Oncol 2009;27:6229-36. [PubMed]
- Hishida T, Nagai K, Mitsudomi T, et al. Salvage surgery for advanced non-small cell lung cancer after response to gefitinib. J Thorac Cardiovasc Surg 2010;140:e69-71. [PubMed]
- Schaake EE, Kappers I, Codrington HE, et al. Tumor response and toxicity of neoadjuvant erlotinib in patients with early-stage non-small-cell lung cancer. J Clin Oncol 2012;30:2731-8. [PubMed]
- Takamochi K, Suzuki K, Sugimura H, et al. Surgical resection after gefitinib treatment in patients with lung adenocarcinoma harboring epidermal growth factor receptor gene mutation. Lung Cancer 2007;58:149-55. [PubMed]
- Hishida T, Nagai K, Mitsudomi T, et al. Salvage surgery for advanced non-small cell lung cancer after response to gefitinib. J Thorac Cardiovasc Surg 2010;140:e69-71. [PubMed]
- Shen H, Zhong X, Ge XQ, et al. Surgical resection of lung adenocarcinoma without EGFR mutation after neoadjuvant gefitinib treatment. Clin Respir J 2010;4:192-3. [PubMed]
- Chaft JE, Oxnard GR, Sima CS, et al. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res 2011;17:6298-303. [PubMed]


