A new addition to the PD-1 checkpoint inhibitors for non-small cell lung cancer—the anti-PDL1 antibody—MEDI4736
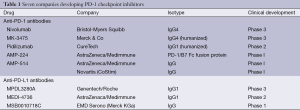
It has been over two years since the phase I studies of the programmed death-1 (PD-1) checkpoint inhibitors—antibodies against PD-1 and its ligand PD-L1—were first presented and published internationally, demonstrating prolonged tumor regressions and improvements in survival (1,2). These results created a paradigm shift in the field of immunotherapy, in that responses were not only observed in melanoma and renal cell cancer, but also in cancers not historically thought to be immunogenic, including lung and ovarian cancer. The past few years have witnessed the rapid development of these agents into phase III registration trials. As shown in Table 1, over seven pharmaceutical companies are now racing to develop and obtain indication of a variety of PD-1 checkpoint inhibitors in a variety of solid tumors. Recently, FDA approval was granted to pembrolizumab (MK-3475) as the first PD-1 checkpoint inhibitor for advanced melanoma, after progression on the cytotoxic T lymphocyte antigen-4 (CTLA-4) inhibitor, ipilimumab (3). However, as the development has been so rapid, there is still little known about the differences between the anti-PD-1 and anti-PDL1 agents developed by the various companies. Immune-related toxicity management algorithms remain in development and the use of immune response criteria is still evolving. Nevertheless, the tolerability and responses seen by these agents have been remarkable. Today, updated phase I trial results for the anti-PD-1 antibody nivolumab (4), the anti-PDL1 antibody MPDL3280A (5), and the anti-PD-1 antibody pembrolizumab (MK3475) (6) leave little lingering doubt that immune checkpoint blockade achieves meaningful and lasting responses in cancer patients, and the list of promising PD-1/PD-L1 inhibitors as well as the list of different tumor types responding to these agents continue to grow.
Full table
A relative newcomer to the field, MEDI4736, has now joined the rapidly expanding body of PD-1 data, with the presentation of its preliminary results from a recent phase 1 study with expansion cohorts in solid tumors (7,8). MEDI4736, a fully-human anti-PD-L1 antibody engineered with a triple mutation in its Fc domain to remove antibody-dependent cell-mediated cytotoxicity, demonstrated an overall response rate across all tumor types of 11% (9/179), 22% among PD-L1+ patients (8/37), and 4% among PD-L1 negative patients (5/113). Within the lung cancer cohort, the overall response was 13%, but up to 39% in PD-L1+ patients and 5% in PD-L1 patients. Based on these outcomes in lung cancer, which aligned with the promising lung cancer data from the other PD-1/PD-L1 phase 1 studies (ORR range, 10-23%), MEDI4736 entered a multi-center, international phase III trial earlier this year for combination therapy with chemoradiation in stage III, unresectable non-small cell lung cancer (clinicaltrials.gov identifier NCT02125461) and a phase II/III trial for recurrent stage IIIB and IV squamous cell lung cancer (NCT02154490).
Preliminary results from the MEDI4736 phase 1 trial in head and neck cancer were also recently presented, showing tumor shrinkage in 7 of 29 evaluable, heavily-pretreated head and neck patients, with none of the responders experiencing relapse yet at 6-24 weeks of follow up (9). Among squamous cell carcinoma of the head and neck, the response rate was 14% (3/22), 50% of PD-L1+ patients (2/4) and 6% (1/16) of PD-L1 negative patients. Responses were seen in other tumor types, but the data is not mature. The disease control rate (RECIST response + stable disease ≥12 weeks) for all tumor types was 31%, 54% (20/37) for PD-L1+ patients, and 21% (24/113) for PD-L1 negative patients, which supports the argument that our traditional measure of response rate does not capture the full efficacy of the checkpoint inhibitors.
MEDI4736 shares many of the hallmarks of its PD-1 competitors: activity across multiple tumor types, rapidity of response with many tumor regressions observed at the first 6-week restaging study, and—most importantly—durability of response, lasting as long as 67 weeks without ongoing treatment. While the PD-L1 status correlated with higher response rates in a variety of tumor types, there were also meaningful responses observed in PD-L1 negative patients, as has been seen in other trials with different PD-1 inhibitors, limiting the reliability of PD-L1 status to determine who should be treated with these agents. It is not yet known if this is due primarily to heterogeneity and nonstandardized methods of immunohistochemical and molecular methods for the current testing for PD-L1 (varying cut-off criteria for positivity, PDL1 status of infiltrating tumor lymphocytes, stromal or tumor cells and specific antibodies used), or if this is due to the unreliability of PD-L1 status itself, which may change in vivo in response to different environmental contexts as well as vary across different tumor sites in the same patient (10).
MEDI4736 may distinguish itself slightly from the other anti-PD-1 agents by its tolerability. Although it is difficult to draw meaningful conclusions from differences across separate trials of the various PD-1 drugs, the toxicities of MEDI4736 were minimally lower than what has been reported with other agents, with only 6% grade 3/4 adverse events in the expansion cohorts, notably no colitis, and only one event of grade 2 pneumonitis, which was reversible (7). MEDI4736 also demonstrated low immunogenicity, with only a 3% incidence (1 in 32 patients) of positive anti-drug antibodies that impacted pharmacokinetic and pharmacodynamics levels (11). This may be a result of MEDI4736 being a fully human antibody, compared to other PD-1 antibodies that contain humanized murine regions which can contribute to variations in affinity and immunogenicity among the PD-1 antibodies. Moreover, it is not known whether the use of an IgG1 or IgG4 backbone elicits any differences in efficacy or tolerability.
The differences between anti-PD-1 antibodies versus those of the anti-PD-L1 antibodies are not well elucidated either. As antibodies specifically targeting PD-L1 such as MPDL3280A and MEDI4736 only block the PD-1: PD-L1 interaction, these agents could theoretically lead to less toxicity than the anti-PD-1 drugs which block both PD-1: PD-L1 and PD-1: PD-L2 binding (12,13). Seemingly minor differences in toxicity patterns become very relevant when considering the distinct tumor types and patient populations that are being targeted for PD-1 development. The toxicity of pneumonitis, for example, can be particularly life threatening in lung cancer where patients often already have compromised lung function from smoking, COPD, radiation history, as well as the cancer itself. However, there is also evidence that some tumors, such as esophageal, hepatocellular and ovarian, express PD-L2, in which case an anti-PD-1 antibody that binds to both PD-L1 and PD-L2 may prove to be more toxic but also more effective (14). It appears some of these antibodies may demonstrate greater efficacy in specific tumor types as opposed to others. For example, MPDL3280A has distinguished itself by its tolerability and efficacy in NSCLC and bladder cancer. It is currently in phase II and III registration trials in lung cancer (NCT02031458, NCT02008227) and in phase II trials in bladder cancer (NCT02108652).
With the presentation of data from each new, successive PD-1 agent, the question remains as to whether the latest PD-1 inhibitor will distinguish itself from its predecessors, and whether one particular agent will prove to be the “winner” in efficacy and side effect profile. MEDI4736 has established itself as a clear contender in the growing family of well-tolerated and promising PD-1 inhibitors, and has entered accelerated development into phase III trials for lung cancer. While there hasn’t been a head-to-head comparison, its preliminary response rates appear at least comparable to the responses observed with other PD-1 inhibitors. It remains to be seen whether the results of these different PD-1 drugs—while promising—reflect a redundancy in anti-tumor activity or whether they will offer a unique therapeutic profile. As we develop a better understanding of the impact of the varying structural elements of these antibodies, and the interactions between PD-1 and PD-L1/L2 on different tumor types, we may find there is not a single winner, but rather that different PD-1 checkpoint inhibitors work better in certain tumor types and for certain patients. As the mechanisms of resistance are investigated, a role may also emerge for the use of these agents in combination with each other and other compounds. For now, the race continues.
Acknowledgements
Dr. Chow is a principal investigator for clinical trials sponsored by AstraZeneca/MedImmune, Genentech, Bristol-Myers Squibb and Merck. She receives advisory board honoraria for Merck and Novartis, and research funding from Pfizer.
Disclosure: The authors declare no conflict of interest.
References
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Press release: FDA approves Keytruda for advanced melanoma. 2014. Available online: www.fda.gov/
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020-30. [PubMed]
- Novello S. Clinical activity, safety and biomarkers of PD-L1 blockade in non-small cell lung cancer (NSCLC): Additional analyses from a clinical study of the engineered antibody MPDL3280A (anti-PDL1). European Cancer Congress 2013:abstr 3408.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 2014;384:1109-17. [PubMed]
- Segal NH, Antonia SJ, Brahmer JR, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. J Clin Oncol 2014;32:abstr 3002.
- Lutzky J, Antonia SJ, Blake-Haskins A, et al. A Phase 1 Study of MEDI4736, an anti–PD-L1 Antibody, in Patients With Advanced Solid Tumors. J Clin Oncol 2014;32:abstr 3001.
- Fury M, Bulter M, Ou SH, et al. Clinical activity and safety of MEDI4736, an anti-PD-L1 antibody, in patients with head and neck cancer. 2014 European Society of Medical Oncology (ESMO) Meeting, Madrid, Spain:abstr 988PD.
- Pardoll D, Drake C. Immunotherapy earns its spot in the ranks of cancer therapy. J Exp Med 2012;209:201-9. [PubMed]
- Fairman D, Narwal R, Liang M, et al. Pharmacokinetics of MEDI4736, a fully human anti-PDL1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol 2014;32:abstr 2602.
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480-9. [PubMed]
- Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy--inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 2012;18:6580-7. [PubMed]
- Rozali EN, Hato SV, Robinson BW, et al. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol 2012;2012:656340.


