
Dynamic changes of acquired T790M mutation and small cell lung cancer transformation in a patient with EGFR-mutant adenocarcinoma after first- and third-generation EGFR-TKIs: a case report
Introduction
Epidermal growth factor receptor (EGFR) inhibitors have revolutionized the treatment of EGFR-mutant non-small cell lung cancer (NSCLC) patients. Unfortunately, all patients will ultimately experience relapse with a median progression-free survival (PFS) of 7 to 16 months during the first- and second-generation EGFR-tyrosine kinase inhibitor (TKI) treatment (1,2). The major resistance mechanism is the EGFR T790M mutation within exon 20, observed in approximately 60% of resistant cases (3,4).
Osimertinib, a third-generation EGFR-TKI, is a pyrimidine-based irreversible inhibitor for both EGFR-activating mutations (e.g., exon 19 deletion or L858R) and T790M mutation. In AURA3, osimertinib was superior to platinum plus pemetrexed in patients with T790M-positive NSCLC who progressed during prior EGFR-TKI therapy, achieving a median PFS of 10.1 months (5). However, similar to first- and second-generation EGFR-TKIs, resistance to osimertinib is inevitable. Numerous resistance mechanisms have been discovered to date, of which transformation to small cell lung cancer (SCLC) has been defined as a rare mechanism occurring in approximately 2% of patients (6,7).
Hence, we report a case in which T790M mutation and SCLC transformation appeared sequentially during treatment with first- and third-generation EGFR-TKIs. We present the following case in accordance with the CARE-Guideline (8).
Case presentation
A 57-year-old never-smoking female complaining with intermittent cough presented in February 2015. Her father had been diagnosed with gastric adenocarcinoma and her sister with breast cancer. Physical examination suggested an Eastern Cooperative Oncology Group (ECOG) performance status of 1, and no significant abnormalities were found. Computed tomography (CT) scans of the chest showed a 20 mm × 14 mm mass in the middle lobe of the right lung, and two small nodules in the right middle lung, and right pleura (Figure 1A). Magnetic resonance imaging (MRI) of the brain and emission computed tomography (ECT) of the bone were also performed, and no other metastases were found. Both trans-bronchial biopsy and pleural lesion biopsy confirmed lung adenocarcinomas (Figure 1B). EGFR exon 19 deletion was identified via the reverse transcription-polymerase chain reaction (RT-PCR) method. So the diagnosis of this patient was stage IV lung adenocarcinoma with an EGFR exon 19 deletion mutation (cT3N0M1a according to TNM version 7).
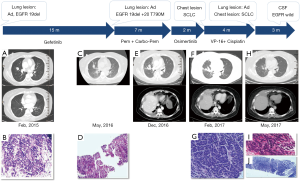
Gefitinib (250 mg daily) was initiated as first-line treatment from March 2015, and the best response was partial response (PR) according to the Response Evaluation Criteria in Solid Tumor version 1.1 (RECIST 1.1). The disease progressed after 14 months, and repeat chest CT scans showed an enlarged right middle lobe mass (Figure 1C). Laboratory findings showed increased carcinoembryonic antigen (CEA) level of 11.1 ng/mL (normal range, 0 to 3.4 ng/mL) and neuron-specific enolase (NSE) of 15.44 ng/mL (normal range, 0 to 12.5 ng/mL) in the serum. A repeat biopsy of the right middle lung mass was performed and histologic analysis showed adenocarcinoma (Figure 1D). The results of immunohistochemistry (IHC) staining were positive for Napsin A, thyroid transcription factor-1 (TTF-1) and cytokeratin 7 (CK-7). Next-generation sequencing including 8 genes (EGFR, ALK, KRAS, ROS-1, BRAF, RET, ERBB2, MET) revealed original EGFR exon 19 deletion and acquired exon 20 T790M mutation.
The third-generation EGFR-TKIs including osimertinib was not accessible in China until 2017, so pemetrexed (500 mg/m2 intravenously on day 1, every 21 days) and carboplatin (AUC 5 intravenously on day 1, every 21 days) were administrated as second-line treatment. The patient achieved a response of PR and a PFS of 5 months. In October 2016, the chest CT scan showed an enlarged right middle lung mass (Figure 1E).
Then this patient was given osimertinib (80 mg daily) as third-line treatment. Regrettably, a mixed response was observed after 2 months, with stable disease of the lung lesions but a sharply increased right lower chest mass (Figure 1F). No metastases were found on brain MRI. The blood test showed significantly increases of NSE (from 24.9 ng/mL in December 2016 to 159.3 ng/mL in February 2017). Biopsy of the progressive chest lesion showed SCLC transformation (Figure 1G). The IHC staining results also showed a neuroendocrine morphology with CD56 and synaptophysin (SyN) positive, which was not seen in pre-osimertinib tumors, while Napsin A, TTF-1 and CK-7 were negative.
For fourth-line treatment, we chose etoposide (100 mg/m2 intravenously on day 1 to 3) plus cisplatin (75 mg/m2 intravenously on day 1, every 21 days), which is the standard regimen for advanced SCLCs. The level of NSE in serum was decreased dramatically to 14.97 ng/mL after two cycles of chemotherapy. The patient achieved a response of PR and a PFS of 4 months (Figure 1H). After progression, we did the fourth biopsy both for the right middle lung mass and the chest mass. Interestingly, the chest mass showed SCLC (Figure 1I), while the histopathology of the lung mass showed adenocarcinoma (Figure 1J). This patient rapidly developed brain metastases in July 2017. No EGFR mutations were detected in her cerebrospinal fluid (CSF). The tolerability of this patient was well during treatment. No severe or unanticipated adverse events were reported. She died in September 2017, and the overall survival (OS) of this patient was 29 months.
Discussion
SCLC transformation is one of the resistance mechanisms associated with first-generation EGFR-TKIs, accounting for about 10% of all cases (7,9). Resistance to osimertinib is also inevitable and numerous mechanisms have been discovered to date. Acquired EGFR C797S mutation was thought to be the major mechanism (10). Meanwhile, SCLC transformation has also been reported as a rare resistance mechanism, accounting for about 2% of cases (6,7). Until now, fewer than 10 SCLC transformation cases after third-generation EGFR-TKIs have been reported in total (11-14). The durations from third-generation EGFR-TKI initiation to transformation is 6 to 18 months, which suggests that long-term exposure to TKI may be needed for SCLC transformation. Furthermore, genomic analysis has shown that the primary EGFR mutation can be universally maintained and the SCLC and EGFR T790M-positive clonal subpopulations seem to be distinct from each other (15).
In our case, we report another metastatic adenocarcinoma patient harboring activating EGFR mutation who experienced T790M mutation and SCLC transformation dynamically after the treatment of first-generation EGFR-TKI gefitinib and third-generation EGFR-TKI osimertinib. Different from previously reported cases, the patient reported in our study experienced rapid SCLC transformation just 2 months after osimertinib initiation. Moreover, the case in our report lost T790M mutation as well as the primary EGFR exon 19 deletion after transformation, which might be a false test result because of the low sensitivity of CSF detection.
The mechanisms of SCLC transformation remain largely unresolved. Several studies have shown that alveolar type II cells may be common precursors of both lung adenocarcinoma and SCLC. Thus, EGFR-mutant lung cancers have the potential to transform into SCLCs during disease progression (16,17). Another hypothesis is that initial tumors consisted of mixed NSCLC and SCLC components. After treatment with EGFR-TKIs, the number of NSCLC decreased and the SCLC became dominant (16). This positive drug selection possibly depends on the tumor microenvironment in lesions in each organ, which results in spatial heterogeneity between lung and chest lesions.
It has been reported that the rapid increase of tumor markers including NSE and pro-gastrin releasing-peptide (pro-GRP) during EGFR-TKI treatment are usually an indication of transformation from NSCLC to SCLC (18-20). In the present case, the remarkably increase in serum levels of NSE highlighted the necessity of repeat biopsy and suggested SCLC transformation (Figure 2).

Recently, a retrospective study examined 58 NSCLC patients diagnosed with adenocarcinoma that transformed to SCLC after one or more EGFR-TKI treatments (21). Median time to transformation was 17.8 months (95% CI, 14.3 to 26.2 months). After transformation, both platinum-etoposide and taxanes yielded high response rates. Median OS after diagnosis was 31.5 months (95% CI, 24.8 to 41.3 months), whereas median survival after the time of SCLC transformation was 10.9 months (95% CI, 8.0 to 13.7 months). In our case, etoposide plus cisplatin chemotherapy yielded considerable response with a response of PR and a PFS of 4 months. The time from diagnosis to transformation was 24 months, and the OS of the patient was 29 months.
In summary, we experienced a case of concomitant mechanisms of drug-resistance via T790M mutation and transformation to SCLC. The dynamic changes among the specific mutations depend on drug-induced selection, which possibly led to drug-resistance. Overall, repeat biopsy is essential for patients whose disease progresses while they are receiving EGFR-TKIs so that appropriate treatment decisions can be made according to the diverse mechanisms of acquired resistance.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ohashi K, Maruvka YE, Michor F, et al. Epidermal growth factor receptor tyrosine kinase inhibitor-resistant disease. J Clin Oncol 2013;31:1070-80. [Crossref] [PubMed]
- Chen D, Chu T, Chang Q, et al. The relationship between preliminary efficacy and prognosis after first-line EGFR tyrosine kinase inhibitor (EGFR-TKI) treatment of advanced non-small cell lung cancer. Ann Transl Med 2019;7:195. [Crossref] [PubMed]
- Lettig L, Sahnane N, Pepe F, et al. EGFR T790M detection rate in lung adenocarcinomas at baseline using droplet digital PCR and validation by ultra-deep next generation sequencing. Transl Lung Cancer Res 2019;8:584-92. [Crossref] [PubMed]
- Ma L, Chen R, Wang F, et al. EGFR L718Q mutation occurs without T790M mutation in a lung adenocarcinoma patient with acquired resistance to osimertinib. Ann Transl Med 2019;7:207. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Ricordel C, Friboulet L, Facchinetti F, et al. Molecular mechanisms of acquired resistance to third-generation EGFR-TKIs in EGFR T790M-mutant lung cancer. Ann Oncol 2018;29:i28-i37. [Crossref] [PubMed]
- Lim SM, Syn NL, Cho BC, et al. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev 2018;65:1-10. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE 2013 Explanations and Elaborations: Reporting Guidelines for Case Reports. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Kim TM, Song A, Kim DW, et al. Mechanisms of Acquired Resistance to AZD9291: A Mutation-Selective, Irreversible EGFR Inhibitor. J Thorac Oncol 2015;10:1736-44. [Crossref] [PubMed]
- Ham JS, Kim S, Kim HK, et al. Two Cases of Small Cell Lung Cancer Transformation from EGFR Mutant Adenocarcinoma During AZD9291 Treatment. J Thorac Oncol 2016;11:e1-4. [Crossref] [PubMed]
- Li L, Wang H, Li C, et al. Transformation to small-cell carcinoma as an acquired resistance mechanism to AZD9291: A case report. Oncotarget 2017;8:18609-14. [PubMed]
- Iijima Y, Hirotsu Y, Mochizuki H, et al. Dynamic Changes and Drug-Induced Selection of Resistant Clones in a Patient With EGFR-Mutated Adenocarcinoma That Acquired T790M Mutation and Transformed to Small-Cell Lung Cancer. Clin Lung Cancer 2018;19:e843-7. [Crossref] [PubMed]
- Ali G, Bruno R, Giordano M, et al. Small cell lung cancer transformation and the T790M mutation: A case report of two acquired mechanisms of TKI resistance detected in a tumor rebiopsy and plasma sample of EGFR-mutant lung adenocarcinoma. Oncol Lett 2016;12:4009-12. [Crossref] [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-72. [Crossref] [PubMed]
- Shi X, Duan H, Liu X, et al. Genetic alterations and protein expression in combined small cell lung cancers and small cell lung cancers arising from lung adenocarcinomas after therapy with tyrosine kinase inhibitors. Oncotarget 2016;7:34240-9. [PubMed]
- Zhang Y, Li XY, Tang Y, et al. Rapid increase of serum neuron specific enolase level and tachyphylaxis of EGFR-tyrosine kinase inhibitor indicate small cell lung cancer transformation from EGFR positive lung adenocarcinoma? Lung Cancer 2013;81:302-5. [Crossref] [PubMed]
- Liu Y. Small cell lung cancer transformation from EGFR-mutated lung adenocarcinoma: A case report and literatures review. Cancer Biol Ther 2018;19:445-9. [Crossref] [PubMed]
- Norkowski E, Ghigna MR, Lacroix L, et al. Small-cell carcinoma in the setting of pulmonary adenocarcinoma: new insights in the era of molecular pathology. J Thorac Oncol 2013;8:1265-71. [Crossref] [PubMed]
- Marcoux N, Gettinger SN, O'Kane G, et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J Clin Oncol 2019;37:278-85. [Crossref] [PubMed]


