
Endocrine adverse events related to immune-oncology agents: retrospective experience of a single institution
Introduction
The advent of the immune-oncology agents (IOA) in the clinical scenario has been a relevant change in the therapeutic approach for several solid tumors (ST), including melanoma, non-small cell lung cancer (NSCLC) and urothelial tumors among others (1,2). These IOA include anti CTLA-4 and anti-PD-1/PD-L1-based immunotherapy and have dramatically changed prognosis of these tumors, with a substantial improvement of overall survival (OS) and with a subset of patients presenting long-lasting responses.
Despite the significant survival benefit in these tumors, as well as an overall better toxicity profile compared to other systemic therapies, IOA present a new spectrum of toxicities. Overall, the boost of the immune system produced by these agents leads to an inflammatory environment in different body tissues, resembling autoimmune disorders, most commonly in the gastrointestinal tract and the skin (3,4). Generally, the incidence of immune-related adverse events (IR-AE) is variable with a mild or moderate symptomatic burden at presentation. Usually, IR-AE can be well controlled with steroids, requiring dose delays and occasionally drug withhold (4). However, IR-AE may be life-threatening with permanent disabling sequels. Their early recognition might help in taking relevant therapeutic measures, guaranteeing treatment adherence by reducing their severity and duration, as well as their impact in patients’ quality of life (5). In addition, the wide spectrum of toxicities related to IOA requires an interdisciplinary approach (6).
Endocrine (E) IR-AE represent the most common form of IR-AE, being thyroid alterations the most prevalent (2). For the present analysis, we sought to characterize the pattern of EIR-AE in a population of patients treated with IOA. We also aimed to correlate their appearance with survival. We hypothesized that EIA-AE occurrence could be associated to a better likelihood of survival in patients receiving IOA, independently of the tumor.
Methods
Patients
All patients with different ST who received treatment with IOA at Catalan Institute of Oncology, Badalona, Hospital Universitari Germans Trias i Pujol from March 2013 to July 2017 were included in the study. Patients with prior conditions or treatments potentially affecting thyroid test results, as steroid treatment or iodine containing contrast, were excluded. None of the patients with prior diabetes has been excluded, since the baseline and subsequent glucose levels were well controlled with the prior medication and no therapeutic modifications were required.
Clinical data was obtained from medical records and included age, gender, tobacco exposure, prior relevant cardiovascular, pulmonary or renal medical history. We also collected date of tumor diagnosis; histological type and stage at diagnosis; and type of prior treatments, as well as molecular data including PD-L1 status analysis and mutational analysis, if available.
IOA included anti-PD-1 (nivolumab, pembrolizumab), antiPDL1 (durvalumab, atezolizumab), anti-CTLA-4 (ipilimumab), or combinations including such drugs. Treatment was maintained until disease progression, loss of clinical benefit or the occurrence of unacceptable toxicity. All patients were followed up until death, withdrawal of consent, or loss of follow-up. If an EIR-AE occurred, patients were referred to the Endocrinology Department for additional assessment and subsequent follow-up.
We monitored thyroid function, including thyroid-stimulating hormone (TSH), triiodothyronine (T3) and free thyroxine (FT4), at baseline and every two cycles during treatment (every 4 weeks for nivolumab and durvalumab, and every 6 weeks for atezolizumab and pembrolizumab regimens, respectively), at least during the first 6 months, and then every 2–3 months. If the patient developed an alteration, we measured them every 6 weeks. We also monitored adrenocorticotropic hormone (ACTH), cortisol, and prolactine (PRL) levels when clinically indicated. Presence of thyroid autoantibodies (Ab), thyroid peroxidase Ab (anti-TPOAb), thyroglobuline Ab (anti-TgAb) and thyroid-stimulating immunoglobulin (anti-TSI) were recommended in patients experiencing thyroid abnormalities. Glycaemia and ionogram were performed at every treatment cycle as part of the routine test. In patients presenting hyperglycemia, pancreatic insulin reserve determined by C-peptide levels and diabetes related Ab (glutamic acid decarboxylase anti-GAD- and insulin Ab-Anti-IA2-) were evaluated. Thyroid ultrasound (US) results were also collected, when available. The diagnosis of hypophysitis was supported radiographically with an MRI of the pituitary gland. Treatment for the EIR-AE included steroids, levothyroxine, antithyroid drugs and insulin, when necessary.
Informed consent was obtained from all individual participants included in the study. Approval was obtained from the Institutional Review Board of our institution (INMUNOEND PROTOCOL 2017).
Statistical analyses
We calculated medians, ranges, frequencies and percentages. Data was additionally stratified by specific tumor type. Overall survival (OS) was defined as the time from the initial diagnosis to death from any cause. Progression-free survival (PFS) was defined as the time from starting IOA to documented disease progression or death. Patients who were still alive at the date of last contact were censored. OS and PFS were calculated with the Kaplan-Meier method. Survival comparisons were calculated with the Log-rank test. We used Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 24.
Results
A total of 260 patients treated with any regimen including IOA from March 2013 to July 2017 were identified. We excluded 72 patients (43% due to prior thyroid disorder, 56.9% due to thyroid dysfunction induced by drugs such as steroids, amiodarone or iodine-containing contrast in the prior 2 months) (Figure 1). One hundred and eighty-eight patients were included in the final analysis.
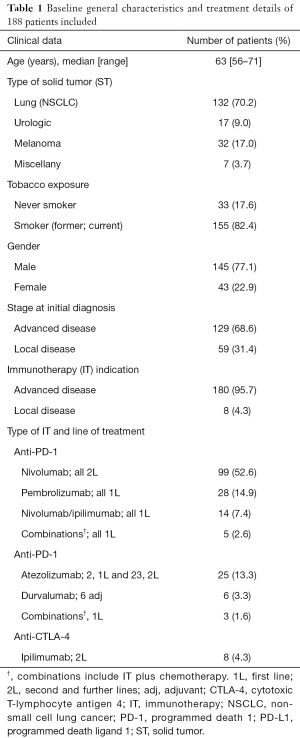
Clinical and molecular characteristics are summarized in Table 1. The median age was 63 years (range, 56–71 years); 77.1% of the patients were male. NSCLC was the most prevalent diagnosis (70.2%) followed by melanoma, urothelial, and others (17.0%, 9.0% and 3.7%, respectively). The 68.6% of tumors were initially diagnosed as advanced disease and IOA was prescribed for advanced disease in 95.7% of the cases.
Full table
Molecular analyses included EGFR, KRAS and BRAF mutations screening as well as ALK rearrangement, ROS translocation and MET amplification for non-squamous lung cancer when available tumor tissue. Analyses were possible in 63.6% of the cases, with a positive result for EGFR, KRAS and BRAF in 3.6%, 16.6% and 2.4%, respectively. No ALK, ROS1 or MET abnormalities were found in this series of lung cancer patients. PD-L1 expression results were available in 40 patients (21.2%) in both squamous and non-squamous lung cancer. PD-L1 expression resulted <1% in 27.5% of the patients and >1% in 72.5% (>50% in 12.5%). Additionally, the presence of BRAF, NRAS and C-KIT mutations were analyzed in patients with melanoma. Results were available for 26 patients with a positive result for BRAF and NRAS in 26.9% and 28.5%, respectively. C-KIT mutations were not detected in this series of melanoma patients.
Nivolumab was prescribed in 52.6% as second line and 7.4% patients as monotherapy and combined with ipilimumab as first line, respectively. Pembrolizumab and atezolizumab were prescribed in 14.9% and 13.3% patients in first line, respectively (Table 1). The median duration of treatment was 4.4 months (range, 1–49.9 months).
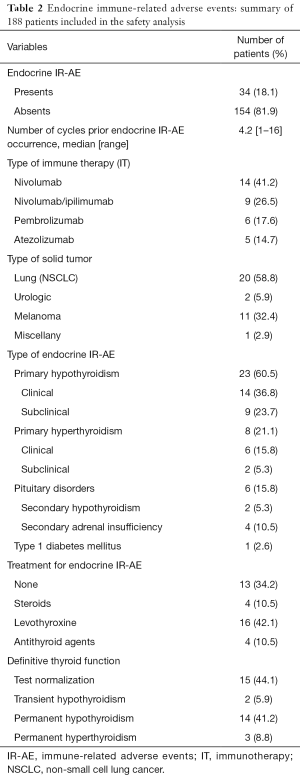
Thirty-four patients (18.1%) developed a total of 38 EIR-AE, the majority of them occurring in those with lung cancer (Table 2). EIR-AE appeared after a median of 4.2 cycles (8 weeks; range, 1–16 cycles). Nivolumab monotherapy or in combination represents the IOA which a higher rate of EIR-AE.
Full table
Primary thyroid alterations were the most common EIR-AE which occurred in 31 (81.6%) of the cases, being hypothyroidism the most prevalent abnormality (n=23; 87.4%) (Table S1). The classical pattern of thyroiditis, with an initial hyperthyroidism phase, was observed only in 7 cases (22.5%), with positive thyroid antibodies and US pattern of thyroiditis in 5 out of 7 cases. Treatment was unnecessary, while spontaneous recovery was observed in all patients in a few weeks. The rest of hypothyroid dysfunctions detected (n=18, 58.1%) were 9 cases (50%) with subclinical hypothyroidism, treated in 5 cases due to elevated THS levels (TSH >10 µUI/mL). The other 9 cases (50%) presented an established hypothyroidism, which required treatment with levothyroxine. Hyperthyroidism was observed in other 8 cases (25.8%), all of them with negative anti TSIAb. Unfortunately, thyroid US was available in only 3 of them, which was consistent with multinodular goiter in 2 cases. Six patients received antithyroid drugs and 2 patients, with subclinical hyperthyroidism, remained untreated at the end of follow up. Pituitary disorders occurred in 6 cases (15.8%), 2 presented secondary hypothyroidism (5.3%) and 4 secondary adrenal insufficiency (10.5%). They received hormone replacement accordingly. Only 1 of them presented an MRI consistent with hypophysitis. One patient (2.6%) developed type 1 diabetes mellitus with a positive anti-GAD-Ab result and C-peptide levels lower than normal. Insulin treatment was started immediately after the diagnosis.

Full table
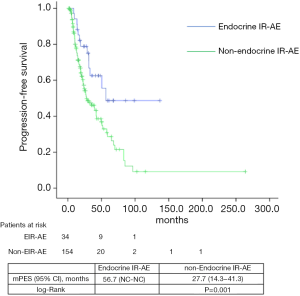
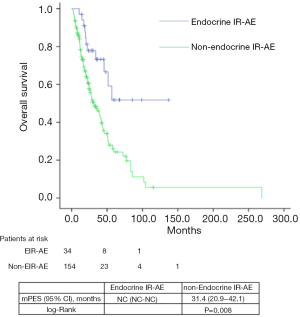
Median PFS and OS for the entire series was 31.1 (24.5–45.7) and 39.7 (29.8–49.6) months, respectively. According to tumor type, median PFS and OS for lung cancer patients were 6.8 (2.5–11.1) and 33.9 (22.7–45.1) months, for urologic patients 6.4 (5.4–7.5) and 26.1 months (16.9–35.3); and for melanoma patients 24.9 (NC–50.9) and 85.6 (24.8–146.3) months. Significant differences were found in both PFS and OS for patients who experienced EIE-AR compared to those who did not [PFS 56.7 (NC–NC) vs. 27.7 (14.3–41.3) months, Log-rank P=0.008; OS NC (NC–NC) vs. 31.4 (20.9–42.1) months, Log-rank P=0.001)] (Figures 1,2). Using a Cox regression model, the development of toxicity was associated with a higher likelihood of survival in the univariate [HR 0.41 (0.22–0.77), P=0.002] and multivariate analysis [HR 0.42 (0.23–0.80), P=0.008]. Except PS at the beginning of the IOA, other factors such as type of tumor, tobacco exposure and stage did not reveal any significant association.
Discussion
In the present study, we report the incidence of the EIR-AE in a series of patients with different ST who received different regimens including IOA. We also report the proposed therapeutic approach and outcome of these EIR-AEs. More interestingly, we present data on clinical outcomes according to the EIR-AE occurrence.
IR-AE can occur with a wide spectrum of toxicities. Data on toxicity comes mainly from the clinical trials, but no specific recommendations for the treatment of such AE are based on prospective evidence, besides expert consensus. The underlying precise pathophysiology of IR-AE is uncertain, since only a small proportion of treated patients presents this toxicity (7-9). Treatment with anti-PD1-PDL1 has been associated with a lower incidence of IR-AE compared to anti-CTLA-4. According to the literature, the incidence of EIR-AE ranges from 0 to 27.8% (10). Frequency of hypothyroidism ranges from 1.5% to 13.6%, hyperthyroidism from 0 to 14% and hypophysitis from in less than 0.1% to 17% (11). Our results are consistent with these previously reported ranges; and thyroid alterations are the most frequent EIR-AE. However, in our series, we excluded patients with prior thyroid abnormalities or thyroid dysfunction induced by drugs such as steroids, amiodarone or iodine-containing contrast. A recent study has also reported thyroid alterations related to IOA by excluding patients with prior thyroid conditions (12). Our exclusion rate was higher (43%), since all the potentially confounding factors have been considered. The final incidence of hypothyroidism has been higher than previously reported once these conditions have been excluded (12).
EIR-AE are likely to occur in any ST treated with any IOA or regimen including such drug, irrespective of the therapeutic line. Incidence of EIR-AE varies widely depending on the studies, since they have been related to both the tumor type and the type of IOA administered (12-14). Our series showed a higher incidence of EIR-AE than previously reported in lung cancer patients, although this tumor is the most prevalent in our series. The incidence of EIR-AE in melanoma patients is higher in our series (15), while in patients with urologic tumors is similar to what has been previously reported (16-18).
Typically, hypothyroidism and hypophysitis are the most common EIR-AE and have been associated with anti-PD-1/PD-L1 and anti-CTLA-4 therapy, respectively (19,20). Our series includes nivolumab and atezolizumab-related pituitary disorders, which has been uncommonly related to anti-PD-1/PD-L1 drugs (19).
A substantial proportion of patients were asymptomatic or paucisymptomatic once the EIR-AE occurred. The similarity with other clinical situations difficult its timely recognition and might partially explain its underreport (19,20). But an early suspicion and differential diagnosis is crucial for an early detection and treatment. Generally, treatment for grade 3 or 4 IR-AE consists of high dose steroids therapy and temporary or permanent interruption of IOA. But, for EIR-AE, hormonal replacement therapy should be recommended for hypothyroidism and secondary adrenal insufficiency and antithyroid drugs should be used for hyperthyroidism. Nevertheless, spontaneous remission thyroid alterations have been reported (27.3% in our series). Very rarely, IOA needs to be discontinued due to an EIR-AE (20). In our series, no patient discontinued treatment.
Onset of EIR-AE may vary according to the type of IOA. In our series, a median of 4.2 cycles which corresponds to approximately 8 weeks of therapy were needed to develop an EIR-AE. This result is consistent with prior data (20). Nonetheless, the possibility of a delayed onset of an EIR-AE should be always considered during the follow-up, even after cessation of IOA treatment (1,8,20).
Some studies have suggested that patients who develop IR-AE are more likely to benefit from immunotherapy (12,15,21-23). But these results have not been widely confirmed. The possibility of certain AE more likely linked to clinical benefit under immunotherapy cannot be excluded, such as vitiligo and anti-CTL4 therapy (24,25). In addition, melanoma patients treated with ipilimumab presenting with a related hypophysitis had a significant longer survival compared to those patients who did not experience it (26,27). This result could be biased by the fact that those patients who live longer are more likely to be exposed to additional doses of ipilimumab, with the additional risk for the IR-AE development. Patients who experienced EIR-AE from our series presented a higher likelihood of survival compared to those who did not. In contrast to prior studies, in which both longer follow-up and a higher exposure to IOA have been suggested as potential explanations for such benefit, in our series, the EIR-AE occurred early (with a median of 4.2 weeks) after the therapy initiation.
There is no current consensus or recommendation about assessment and management of endocrine toxicity beyond baseline and periodic hormonal tests. In general, a multidisciplinary approach to detect and timely treat any related AE is generally accepted (26). For the clinical daily practice, we find of especial interest a prior proposed algorithm that includes systematic hormonal evaluation (28). Thyroid function tests (TSH, T3 and FT4) should be evaluated baseline and periodically. An early referral should be considered in case of any abnormality is detected. Evaluation of thyroid autoAb and thyroid US might be considered in the work-up process and etiologic diagnosis for those patients developing any thyroid abnormality. Since thyroid dysfunction, adrenal insufficiency and diabetes mellitus are the most common and life-threatening endocrine toxicities, we also recommend performing fasting plasma glucose and cortisol measurement before initiating treatment with IOA. In our series, EIR-AE appeared after a median of 8 weeks of therapy, therefore we propose measurement of FT4 once every 2 weeks and TSH every 4 weeks in the two first months of treatment. Biweekly monitoring of TSH levels might not add any benefit, since TSH levels lag behind changes in serum thyroid hormones levels. Subsequently, the time required to reach stable TSH levels according to FT4 levels ranges from 4 to 8 weeks (29). Glucose levels should be evaluated before each cycle. Suspicion of endocrine dysfunction should prompt a new test.
Conclusions
In conclusion, EIR-AE, especially thyroid abnormalities, occur in a significant proportion of patients treated with IOA. As other IR-AE, EIR-AEs require a multidisciplinary approach. Their early detection is crucial for an early referral and therapy initiation, when necessary, to guarantee treatment adherence and to minimize the impact in patients’ quality of life. In addition, patients who develop EIR-AE might associate a better prognosis compared to those who do not experienced such toxicity.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent for data collection and publication was obtained from all individual participants included in the study. Approval was obtained from the Institutional Review Board of Hospital Universitari Germans Trias I Pujol (INMUNOEND PROTOCOL 2017).
Data Sharing Statement: No additional data available.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Nishino M, Ramaiya NH, Hatabu H, et al. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 2017;14:655-68. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Haanen JBAG, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 2017;28:iv119-42. [Crossref] [PubMed]
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559-74. [Crossref] [PubMed]
- O’Kane GM, Labbé C, Doherty MK, et al. Monitoring and Management of Immune-Related Adverse Events Associated With Programmed Cell Death Protein-1 Axis Inhibitors in Lung Cancer. Oncologist 2017;22:70-80. [Crossref] [PubMed]
- Byun DJ, Wolchok JD, Rosenberg LM, et al. Cancer immunotherapy — immune checkpoint blockade and associated endocrinopathies. Nat Rev Endocrinol 2017;13:195-207. [Crossref] [PubMed]
- Krummel MF. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med 1996;183:2533-40. [Crossref] [PubMed]
- Nagai H, Muto M. Optimal management of immune-related adverse events resulting from treatment with immune checkpoint inhibitors: a review and update. Int J Clin Oncol 2018;23:410-20. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- González-Rodríguez E, Rodríguez-Abreu D. Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016;21:804-16. [Crossref] [PubMed]
- Cukier P, Santini FC, Scaranti M, et al. Endocrine side effects of cancer immunotherapy. Endocr Relat Cancer 2017;24:T331-47. [Crossref] [PubMed]
- Remon J, Pardo N, Martinez-Martí A, et al. Immune-checkpoint inhibition in first-line treatment of advanced non-small cell lung cancer patients: Current status and future approaches. Lung Cancer 2017;106:70-5. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft J, et al. Antibody-Mediated Thyroid Dysfunction During T-cell Checkpoint Blockade in Patients with Non-Small Cell Lung Cancer. Ann Oncol 2017;28:583-9. [Crossref] [PubMed]
- Remon J, Mezquita L, Corral J, et al. Immune-related adverse events with immune checkpoint inhibitors in thoracic malignancies: focusing on non-small cell lung cancer patients. J Thorac Dis 2018;10:S1516-33. [Crossref] [PubMed]
- Weber JS, Hodi FS, Wolchok JD, et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients With Advanced Melanoma. J Clin Oncol 2017;35:785-92. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res 2015;4:560-75. [PubMed]
- Shang YH, Zhang Y, Li JH, et al. Risk of endocrine adverse events in cancer patients treated with PD-1 inhibitors: a systematic review and meta-analysis. Immunotherapy 2017;9:261-72. [Crossref] [PubMed]
- Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139-48. [Crossref] [PubMed]
- Krummel MF, Allison JP. CTLA-4 Engagement Inhibits 11.+-2 Accumulation and Cell Cycle Progression upon Activation of Resting T Cells By Matthew E Krummel and James P. Allison. J Exp Med 1996;183:2533-40. [Crossref] [PubMed]
- Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci 2003;100:8372-7. [Crossref] [PubMed]
- Downey SG, Klapper JA, Smith FO, et al. Prognostic Factors Related to Clinical Response in Patients with Metastatic Melanoma Treated by CTL-Associated Antigen-4 Blockade. Clin Cancer Res 2007;13:6681-8. [Crossref] [PubMed]
- Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152:45-51. [Crossref] [PubMed]
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-Like Depigmentation in Patients With Stage III-IV Melanoma Receiving Immunotherapy and Its Association With Survival: A Systematic Review and Meta-Analysis. J Clin Oncol 2015;33:773-81. [Crossref] [PubMed]
- Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J Clin Endocrinol Metab 2014;99:4078-85. [Crossref] [PubMed]
- Illouz F, Briet C, Cloix L, et al. Endocrine toxicity of immune checkpoint inhibitors: essential crosstalk between endocrinologists and oncologists. Cancer Med 2017;6:1923-9. [Crossref] [PubMed]
- Kohler S, Senn O, Saleh L, et al. Timing of Thyroxine Dose Adjustment in Hypothyroid Patients: When are TSH Levels Stable? Thyroid Disord Ther 2014;3:161.


