
Long-term survival with targeted therapy in an advanced non-small cell lung cancer patient based on genetic profiling
Introduction
The discovery of epidermal growth factor receptor (EGFR) mutations and their sensitivity to EGFR inhibitors have dramatically altered the treatment of patients with non-small cell lung cancer (NSCLC) (1,2). Furthermore, multi-generations tyrosine kinase inhibitors (TKI) against EGFR-driven mutations have produced significant effects on NSCLC patients (3-5). Targeted therapy though, is similar to chemotherapy and is facing problems of resistance. The underlying mechanisms of acquired resistance (AR) to EGFR-TKIs are also complex. As such, there may be added factors other than EGFR mutations that contribute to its disease progression (6-8). Thus further research needs to be carried out to find new targets. The administration of these targeted drugs, dynamic monitoring of genomic profiles during treatment, and the promotion of targeted therapy all depend on the extensive application of next-generation sequencing (NGS) in tissue biopsy or liquid biopsy.
In this report, we regularly used tissue biopsies or liquid biopsies to monitor a patient with EGFR-exon19del positive NSCLC who has at present achieved overall survival for up to 48 months through dynamic monitoring of genomic profile during cancer processes.
Case presentation
Patient management is described in Figure 1A. A 32-year-old woman with no prior history of smoking was referred to another hospital complaining of cough accompanied with numbness of the right upper limb and face, mild headache, and dizziness in November 2015. The systematic evaluation indicated that a stage IV T3N0M1 poorly differentiated NSCLC accompanying with bone metastasis and intracranial metastasis were involved. The examination of EGFR mutations by amplification refractory mutation system demonstrated EGFR with exon 19 deletion mutations.
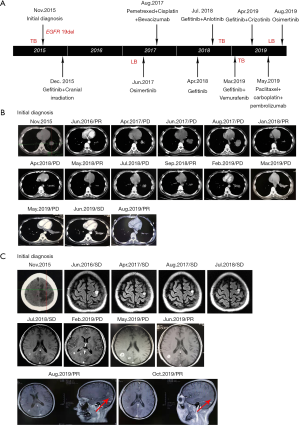
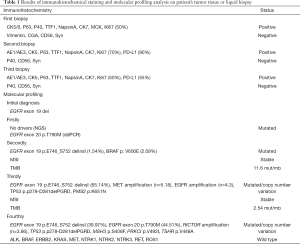
Beginning on December 1, 2015, this patient orally received gefitinib treatment plus whole-brain radiotherapy PTV 30 Gy/10 F. 4mg zoledronic acid intravenous drip regularly was used for bone treatment. On June 18, 2016, a chest and abdomen computed tomography (CT) scan showed a significant reduction in primary lesions (Figure 1B). Efficacy was assessed as partial remission (PR). And brain magnetic resonance imaging (MRI) showed stable disease (SD) in metastases (Figure 1C). After a year of continued gefitinib treatment, chest-abdomen CT showed progressive disease (PD) (Figure 1B). Blood NGS testing was subsequently performed, but no EGFR driving genes and other positive driving genes were found, while ddPCR indicated EGFR 20 exon T790M mutation (Table 1). Thus, osimertinib targeted therapy was selected for two months, but no significant effect.
Full table
The patient was moved to our unit on 31 August 2017 after undergoing the above treatment. A treatment regimen of pemetrexed/ cisplatin with bevacizumab was firstly administered in 4 cycles. The partial regression treatment effect was obtained (Figure 1B), and the brain MRI showed a stable lesion (Figure 1C). However, the disease progressed after two more cycles of chemotherapy. Re-challenge with gefitinib was selected since April 2018. Unfortunately, the disease progressed again after a brief remission (Figure 1B). Therefore, a rebiopsy was used to guide further treatment. And gefitinib plus anlotinib was performed as the next treatment. The lesion was diminished but enlarged again. Then the targeted therapy with gefitinib plus vemurafenib was started while targeted NGS found EGFR exon 19 p.E746_S752 delinsI (1.54%) and BRAF V600E mutation (2.09%) (Table 1). The size of the primary lung tumor was increased after a month of treatment. Subsequently, a third biopsy was performed on March 27, 2019. And NGS revealed EGFR exon 19 p.E746_S752 delins I (39.97%), MET amplification (n=5.18) and EGFR amplification (n=4.3). So the treatment regimen was switched to gefitinib plus crizotinib. However, the patient complained of dizziness, the right eye was diplopia, and the right eyelid was drooping by May 2019. Challenge with Paclitaxel /carboplatin plus pembrolizumab was selected for 2 cycles followed. Surprisingly, there was an obvious improvement in tumor lesions (Figure 1B), no obvious abnormality in the right eyelid, no obvious headache or dizziness, and diplopia disappeared. Undesirably, the patient developed hyperthyroidism with palpitations, irritability, and prominent eyes. The PS of the patient worsens from 1 to 2 with obvious poor appetite, weight loss, slight headache, and weakness again. On Augest10, 2019, the NGS-based genotyping was performed on cerebrospinal fluid (CSF) derived circulating tumor DNA (ctDNA). EGFR exon 19 p.E746_S752 delinsI (65.14%), EGFR exon 20 p.T790M (44.51%) and RICTOR amplification (n=3.66) were observed. And the patient is currently receiving treatment with osimertinib.
Discussion
In the past decade, considerable progress has been made in the treatment of advanced NSCLC, especially molecular targeted therapy. Regardless of the technique used, the determination of tumor molecular status has become the standard for NSCLC treatment. The EGFR signaling pathway plays a crucial role in the NSCLC development and progression, and EGFR mutation sensitization is found in approximately 10–15% of white patients and up to 50% of Asian NSCLC patients (9,10). Here, we report an advanced NSCLC Chinese patient with EGFR 19 deficiency who was initially treated with gefitinib orally and responded to PR. Numerous studies (11,12) have shown that first and second-generation EGFR TKIs such as erlotinib, gefitinib, or afatinib were recommended for patients with advanced NSCLC who have EGFR mutation activation (exon 19 deletion and 21 L858R point mutation). However, NSCLC invariably produces AR to first-line inhibitors. Most potential AR mechanisms have been identified, including secondary mutations in EGFR (e.g., EGFR-T790M), activation of bypass signaling pathways (such as BRAF, MET, PIK3CA signaling pathway), and histological transformation to small cell lung cancer (13). To our knowledge, this report is the first to show a patient received the ninth-line therapy who underwent multiple biopsies, including tissue biopsy and liquid biopsy, and then targeted therapy based on corresponding different AR mechanisms to achieve an overall survival currently up to 48 months.
A previous Phase II Study had indicated that gefitinib re-challenge was effective as an option after the first-line EGFR-TKI treatment and second-line chemotherapy, especially for the T790M-negative patients (14). Here, a similar treatment regimen was performed, while progression continued again after patients received first line gefitinib and third-line chemotherapy, although EGFR T790M mutation was detected by ddPCR not in blood NGS before. And there was also no response to second line osimertinib, suggesting that it was likely to be a false positive mutation, and blood NGS results seem more reliable. Compared with the traditional detection methods, the sensitivity of NGS has been significantly improved with a reported detection sensitivity of 0.1–1.0%. And it can detect multiple genes simultaneously, including point mutation and insertion/deletion, copy number variation and chromosome rearrangement (15). Thus, more comprehensive and precise AR mechanisms have been discovered. In this study, an alternative arrangement of resistance such as BRAF V600E mutation, MET amplification, and EGFR T790M mutation was detected. Noteworthily, a RICTOR amplification was found finally in the CSF using NGS test, which occurs in 13% (132/1,016) of patients with lung cancers from the TCGA database. And another independent series revealed that 8% (85/1,070) of lung cancer patients with RICTOR amplification. Of these, 26% (22/85) of these patients had alterations in EGFR (16-19).
Interestingly, RICTOR amplification is associated with sensitivity to mTOR1/2 inhibitors. The index lung cancer patient was stable after receiving treatment with mTOR1/2 inhibitors (20), whether it can provide a reference for the next treatment plan for the patient in our study.
Besides, as of October 2016, the FDA approved the first line pembrolizumab for PD-L1 high-expression tumor patients with a score of ≥50%. At present, the examination of tumor PD-L1 level was also considered the standard of care for advanced metastatic NSCLC. Interestingly, our case receiving multiple rebiopsies showed dynamism in PD-L1 expression. Similar to previous cases, suggesting that PD-L1 expression may also be affected by EGFR-TKIs, just as T790M status was affected by EGFR-TKI exposure (21,22). Our results from multiple biopsies also varied. It may due to that PD-L1 expression is spatially heterogeneous (23), or its expression can also be regulated by EGFR-TKIs treatment (24,25). Therefore, multiple biopsies may be desirable to examine PD-L1 expression as a predictor of immunotherapy or explore AR mechanisms such as T790M status. In this case report, the first two tissue biopsies of NGS did not reveal the T790M mutation, whereas the recent CSF NGS test showed a T790M mutation. The missing of T790M mutation in tumor tissue may be due to limited tissue available from the lung as well as inter- and intra-tumor heterogeneity, where the tissue biopsy does not provide a comprehensive understanding of the genetic heterogeneity of the entire tumor or metastatic disease (26). Of course, the progression of alternative resistance mechanisms may also lead to such results. Meanwhile, the T790M mutation detected in liquid biopsy may be due to the nature of the metastatic disease and the diversity of ctDNA population, which represents the overall mixed heterogeneity of the disease rather than being confined to a single small tissue sample (26). On that basis, we were able to make an informed clinical decision to start using osimertinib, and patients responded.
In summary, this case gave us some important hints for early NSCLC diagnosis and treatment. Firstly, biomarker analysis should be regularly considered part of the resistance mechanism and initial molecular diagnosis, particularly with NGS technique. Secondly, liquid biopsy, including CSF liquid biopsy, could be a promising method for the diagnosis of resistance mechanisms with metastatic disease. Finally, the dynamic monitoring of the tumor genome by multiple biopsies can name tumor driving genes and drug resistance mechanisms to guide tumor treatment.
Acknowledgments
The authors thank Shanghai Tongshu Biotechnology Co., Ltd. for technical support.
Funding: This work was supported by the Wujieping Medical Foundation (320.6750.13306).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.01.21). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The Research Ethics Committee approved this study of Shanxi Provincial Cancer Hospital. The written informed consent was obtained from the patient for specimen collection, genetic testing, and her information used for research.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fassunke J, Muller F, Keul M, et al. Overcoming EGFR(G724S)-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun 2018;9:4655. [Crossref] [PubMed]
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Karachaliou N, Fernandez-Bruno M, Bracht JW, et al. EGFR first- and second-generation TKIs—there is still place for them in EGFR-mutant NSCLC patients. Transl Cancer Res 2019;8:S23-47. [Crossref]
- Clinical Lung Cancer Genome P. Network Genomic M. A genomics-based classification of human lung tumors. Sci Transl Med 2013;5:209ra153. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Ma L, Chen R, Wang F, et al. EGFR L718Q mutation occurs without T790M mutation in a lung adenocarcinoma patient with acquired resistance to osimertinib. Ann Transl Med 2019;7:207. [Crossref] [PubMed]
- Hong S, Gao F, Fu S, et al. Concomitant Genetic Alterations With Response to Treatment and Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With EGFR-Mutant Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:739-42. [Crossref] [PubMed]
- Lettig L, Sahnane N, Pepe F, et al. EGFR T790M detection rate in lung adenocarcinomas at baseline using droplet digital PCR and validation by ultra-deep next generation sequencing. Transl Lung Cancer Res 2019;8:584-92. [Crossref] [PubMed]
- Hirsch FR, Bunn PA Jr. EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009;10:432-3. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013;105:595-605. [Crossref] [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [Crossref] [PubMed]
- Song Y, Wu YL, Cao LJ, et al. Efficacy and Safety of Gefitinib as Third-line Treatment in NSCLC Patients With Activating EGFR Mutations Treated With First-line Gefitinib Followed by Second-line Chemotherapy: A Single-Arm, Prospective, Multicenter Phase II Study (RE-CHALLENGE, CTONG1304). Am J Clin Oncol 2019;42:432-9. [Crossref] [PubMed]
- Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet 2016;17:333-51. [Crossref] [PubMed]
- Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell 2012;150:1107-20. [Crossref] [PubMed]
- Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [Crossref] [PubMed]
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401-4. [Crossref] [PubMed]
- Cheng H, Zou Y, Ross JS, et al. RICTOR Amplification Defines a Novel Subset of Patients with Lung Cancer Who May Benefit from Treatment with mTORC1/2 Inhibitors. Cancer Discov 2015;5:1262-70. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Hata A, Katakami N, Yoshioka H, et al. Spatiotemporal T790M Heterogeneity in Individual Patients with EGFR-Mutant Non-Small-Cell Lung Cancer after Acquired Resistance to EGFR-TKI. J Thorac Oncol 2015;10:1553-9. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol 2014;25:1935-40. [Crossref] [PubMed]
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63. [Crossref] [PubMed]
- Mambetsariev I, Vora L, Yu KW, et al. Effective osimertinib treatment in a patient with discordant T790 M mutation detection between liquid biopsy and tissue biopsy. BMC Cancer 2018;18:314. [Crossref] [PubMed]


