
Comparison of autofluorescence and white-light bronchoscopies performed with the Evis Lucera Spectrum for the detection of bronchial cancers: a meta-analysis
Introduction
Lung cancer is a major cause of cancer-related death worldwide (1,2). It was reported that the 5-year survival rate for those patients with a stage 0 cancer is more than 90% (3), whereas the rate for patients with stage IA disease is 73%, and the rate for stage II to IV ranges from 9% to 46% (4). Therefore, it is essential to clinically detect early lung cancers by using more sensitive methods, as the discovery and treatment of early stage lung cancer not only enhances the survival rate, but also the patients’ quality of life (5-7). This goal can only be achieved with the development of more sensitive methods that have an acceptable specificity for these early stages. Interestingly, the development of these newer technologies has also provided useful information to better understand tumor transformation and the carcinogenic mechanisms of bronchial (pre) cancerous lesions (8).
A number of studies have compared the sensitivity and specificity of autofluorescence bronchoscopy (AFB) and white-light bronchoscopy (WLB) (3,9,10). The results and conclusions were discordant, a situation which, unfortunately, contributed to the limited acceptance of AFB (10-16). In order to solve this problem, Chen et al. used a meta-analysis which demonstrated that AFB had a higher sensitivity and lower specificity, and that the combined utilization of AFB and WLB was superior to WLB alone and had a higher sensitivity; there was, however, no analysis comparing the specificity of these methods performed in this study (17).
Autofluorescence imaging (AFI) is a term adopted by the Olympus Medical Systems Corporation to describe AFB based on its technology. Briefly, the AFI system developed by this company (Evis Lucera Spectrum) consists of three main parts (for more details, please visit: https://www.olympus-global.com/en/news/2006a/nr060515evise.html): a xenon light source, an autofluorescence video bronchoscope (BF-F260), and a video processor unit (CV-260SL). Images produced by AFI technology can be displayed in both the traditional (white light) and autofluorescence modes on the same monitor via a switch. The system transmits 3 wavelengths: excitation blue light (395–445 nm, to induce autofluorescence), 550 nm (red reflected light), and 610 nm (blue reflected light). Normal mucosa appears green, inflammation appears blue (because of a high concentration of hemoglobin which can absorb the green and red wavelengths), and cancers and precancerous lesions appear magenta (because they can mix red/blue signals and shorten the green autofluorescence) (18,19).
In the present study, we explored the reported performance of AFI compared to WLB in the diagnosis of bronchial cancerous lesions.
Methods
Search strategy
We conducted a systematic search of the PubMed, Embase, Web of Science and CNKI databases, from inception to July 12th, 2018; we restricted our search to English language publications to avoid sources of local/national articles which are frequently of low quality. The following keywords were used as search terms: (“optical imaging”[MeSH Terms] OR (“optical”[All Fields] AND “imaging”[All Fields]) OR “optical imaging”[All Fields] OR (“autofluorescence”[All Fields] AND “imaging”[All Fields]) OR “autofluorescence imaging”[All Fields]) AND videobronchoscopy[All Fields] and white-light[All Fields] AND (“bronchoscopy”[MeSH Terms] OR “bronchoscopy”[All Fields]).
Inclusion and exclusion criteria
Our inclusion criteria for studies were as follows:
- Involved patients who were suspected of having bronchial cancer;
- Compared the use of an AFI system with WLB bronchoscopy;
- Used histological analysis of biopsies as the golden standard for diagnosing bronchial cancer, with the status of positive results for “moderate dysplasia or worse” or “mild dysplasia or worse” or “tumor” in different studies. The detailed characteristics of the included studies are shown in Table 1.
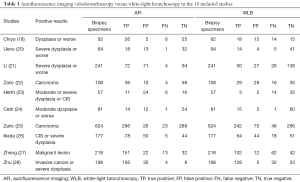
Full table
Studies were excluded if they were duplicate studies or in vitro studies, involved animal experiments, lacked a control group to compare the capabilities of WLB with AFI, or if the identified study was a meeting abstract.
Quality assessment and data extraction
Data were extracted independently by two investigators, and differences were resolved by consensus. Quality assessment of these studies was performed using Cochrane Collaboration’s risk-of-bias tool which considers the following criteria: reporting of randomization method, allocation concealment, blinding of outcome assessment, completeness of follow-up, and bias of selective reporting (29).
Statistical analysis
The random model for the diagnostic meta-analysis was used to obtain pooled sensitivities and specificities (30), with pooled sensitivity and specificity of AFI and WLB being estimated as diagnostic capability, which were displayed in a forest plot. The positive predictive value, negative predictive value, and the area under the curve (AUC) were analyzed simultaneously. P<0.05 was used to identify significant differences.
The two investigators constructed 2×2 tables for each study. The contents of the four table cells were as follows: true positive (TP), false positive (FP), false negative (FN), and true negative (TN). We used STATA 14.0 (StataCorp, College Station, TX, USA), in particular the MIDAS Command Language, for all statistical analysis. The sensitivity was identified as the percentage of the disease which was diagnosed correctly according to the criteria of the screening method. The specificities were identified as the percentage of the actual disease which was not diagnosed according to the method.
Results
Literature search results and population characteristics
Using the methods described above, we identified 189 publications which were selected by our filtration criteria. Of these, 33 duplicates and 76 other articles were excluded (animal experiments, in vitro studies, multiple subjects, and meeting abstracts), leaving 80 articles. A further 46 studies were excluded after a careful review of the titles and abstracts revealed that they were not comparisons or were not relevant to the present study.
After screening for articles of high-quality that also met our specific inclusion criteria, a total of 10 articles (18,20-28) were eligible for the final meta-analysis. A flow chart of our meta-analysis is presented in Figure 1, while detailed features of the included studies are presented in Table 2.
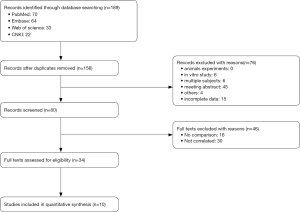
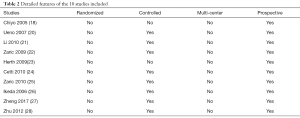
Full table
Quality assessment
As shown in Figure 2, the quality of the studies included in the present investigation was assessed by the Cochrane risk-of-bias tool. Some studies failed to provide a clear method of blinding (including the blinding of participants, personnel, and outcome assessment), while a few studies had limitations in sample size.
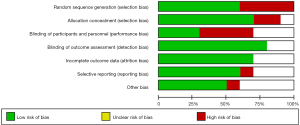
Diagnostic accuracy indices
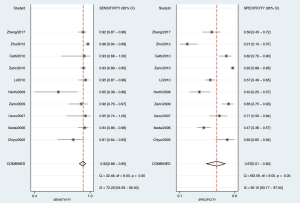
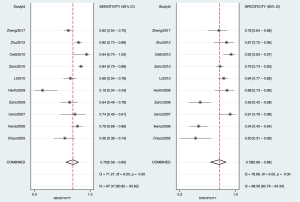
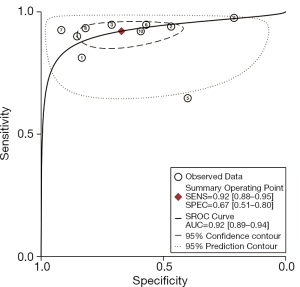
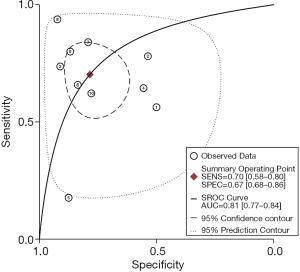
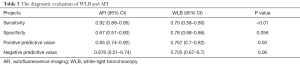
As shown in Figure 3, the sensitivity of AFI ranged from 0.65 to 0.98, with an I2 of 72.29 (range, 54.59–90.00), while the specificity varied between 0.21 and 0.92, with an I2 of 95.10 (range, 93.17–97.02). As shown in Figure 4, the sensitivity of WLB ranged from 0.18 to 0.94, with an I2 of 87.37 (range, 80.82–93.92), and the specificity ranged from 0.50 to 0.92, with an I2 of 88.56 (range, 82.79–94.33). The pooled sensitivity and specificity of AFI were 0.92 (95% confidence interval, 0.88–0.95) and 0.67 (95% confidence interval, 0.51–0.80), respectively (Figure 3). The pooled sensitivity and specificity of WLB were 0.70 (95% confidence interval, 0.58–0.80) and 0.78 (95% confidence interval, 0.68–0.86), respectively (Figure 4). The positive predictive value of AFI vs. WLB was 85.0% vs. 76.7% respectively (P<0.05), and the negative predictive value of AFI vs. WLB was 67.6% vs. 70.5% (P=0.06), respectively. Our study showed that the AUCs of AFI and WLB were 0.92 (range, 0.89–0.94) and 0.81 (range, 0.77–0.84), respectively (Figures 5 and 6 for AFI and WLB, respectively). Chi-square test was used to compare the difference between the two rates, and the specificity of AFI vs. WLB showed no difference (P=0.056). There was no difference of the negative predictive value between AFI and WLB (P=0.06) (data available in Table 3).
Full table
Publication bias and stability of the results
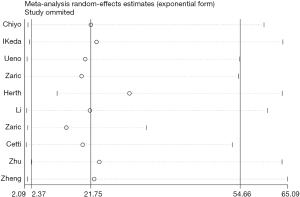
The Egger’s test used to assess publication bias resulted in a P value of 0.225 (Table 4), which suggested there was no or little publication bias. Sensitivity analysis (Figure 7) was used to assess the stability of the results; the 95% confidence intervals of each trial overlapped with one another, proving our eligible stability.

Full table
Discussion
After passing this review’s strict selection criteria, 10 articles were eligible for the final meta-analysis. A total of 1,830 patients were included in the analysis. We found a better relative sensitivity and a comparable specificity of AFI versus WLB by comparing various indicators of diagnostic effectiveness. Subsequent objective evaluation of the publication bias and the stability of our results indicate that our results are likely to be credible.
Zheng et al. (27) have previously described the factors explaining AFI’s poorer specificity when compared to WLB: the friction damage of the airway wall caused by bronchoscopy in the process, airway mucosal inflammation, oral anticoagulation, ingestion of photosensitizing drugs within 3 months, cytotoxic chemotherapy conducted within 6 months, and many nonneoplastic diseases leading to false-positive results in AFI. This study is an impetus to research more effective image analysis methods and to optimize the spectral design of AFI. Indeed, it should be noted that the results reported above are specific for the Evis Lucera Spectrum from Olympus, whereas other manufacturers are commercial systems based on significantly different spectral designs to perform AFB. Sutedja (31) showed that by using AFI and combining methods of forceps biopsy, brush biopsy, needle aspiration, and douche to acquire samples, the comprehensive positive diagnosis rate of lung cancer was clearly improved, demonstrating that AFI has more significant clinical value than WLB for the diagnosis of bronchial cancer. Other studies and meta-analyses have concluded that AFB has a higher sensitivity and lower specificity than WLB (20-22,28), and some studies proved that the usage of AFI does not increase the adverse effects of bronchoscopy (23,25).
Some limitations existed in our research. First, there was an absence of relevant, large randomized controlled trials. Second, we did not investigate the different pathological types of lung cancers, such as mild-to-moderate, moderate-to-severe, and severe types. Instead, we roughly divided the pathological types into a normal group and a malignant cancer group. Third, the level of training (learning curve) of the bronchoscopists was not taken into account. Fourth, since virtually all biopsies were taken under white light observation, the spatial precision of the tissue uptake was probably much better for WLB than AFI, a bias which precludes the assessment of the performances of the latter method. Furthermore, it was not possible to completely rule out sources of publication bias such like incomplete data and inconsistent positive results.
More refined and extended versions of such analysis would also provide interesting information for the medical, industrial, and scientific communities active in the field of AFB. In particular, assessing the performance of AFI used in combination with WLB and/or narrow band imaging (NBI) would be interesting. Also, a comparison of the performances achieved with different commercially available systems for AFB would enable us to identify which generations and, importantly, which spectral designs (excitation and detection wavelengths, combined detection of autofluorescence, and backscattered light at specific wavelengths) are optimal for the detection and/or demarcation of bronchial cancers. Indeed, these spectral designs vary significantly across these systems, with some of them being particularly optimized to minimizing false positives, probably without affecting their sensitivities (32-34).
Finally, one interesting general consensus revealed from the analysis of the articles considered in our meta-analysis is that AFI should be used for detection purposes in patients in whom pre-invasive lesions (dysplastic, carcinoma in situ) have been detected but who have showed no evidence of invasive cancer. In addition, AFI should be used for demarcation purposes in patients with early invasive lung cancers for whom endobronchial therapy is indicated.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (grant number 81401903, 81572937 and 81572273); the16th batch “Summit of the Six Top Talents” Program of Jiangsu Province (grant number WSN-154); China Postdoctoral Science Foundation 12th batch Special fund (Postdoctoral number: 45786); China Postdoctoral Science Foundation 64th batch (Postdoctoral number: 45786); Jiangsu Provincial Postdoctoral Science Foundation (grant number 2018K049A); the Natural Science Foundation of Jiangsu province (grant number BK20180139 and BK20161386 and BK20191351); Jiangsu Provincial Medical Youth Talent (grant number QNRC2016125), and the Nanjing Medical Science and Technology Development Project (No. ZKX17044), and Jiangsu Planned Projects for Postdoctoral Research Funds (2019k178).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Sharing Statement: No additional data available.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220-41. [Crossref] [PubMed]
- Lancia A, Merizzoli E, Filippi AR. The 8th UICC/AJCC TNM edition for non-small cell lung cancer staging: getting off to a flying start? Ann Transl Med 2019;7:S205. [Crossref] [PubMed]
- Lam S, MacAulay C, leRiche JC. Detection and localization of early lung cancer by fluorescence bronchoscopy. Cancer 2000;89:2468-73. [Crossref] [PubMed]
- Hernandez BY, Green MD, Cassel KD, et al. Preview of Hawaii Cancer Facts and Figures 2010. Hawaii Med J 2010;69:223-4. [PubMed]
- Tseng TS, Gross T, Celestin MD, et al. Knowledge and attitudes towards low dose computed tomography lung cancer screening and smoking among African Americans—a mixed method study. Transl Cancer Res 2019;8:S431-42. [Crossref]
- Revelo AE, Martin A, Velasquez R, et al. Liquid biopsy for lung cancers: an update on recent developments. Ann Transl Med 2019;7:349. [Crossref] [PubMed]
- Tu H, Wu M, Huang W, Wang L. Screening of potential biomarkers and their predictive value in early stage non-small cell lung cancer: a bioinformatics analysis. Transl Lung Cancer Res 2019;8:797-807. [Crossref] [PubMed]
- Uehlinger P, Gabrecht T, Glanzmann T, et al. In vivo time-resolved spectroscopy of the human bronchial early cancer autofluorescence. J Biomed Opt 2009;14:024011. [Crossref] [PubMed]
- Lam S, leRiche JC, Zheng Y, et al. Sex-related differences in bronchial epithelial changes associated with tobacco smoking. J Natl Cancer Inst 1999;91:691-6. [Crossref] [PubMed]
- Ikeda N, Honda H, Katsumi T, et al. Early detection of bronchial lesions using lung imaging fluorescence endoscope. Diagn Ther Endosc 1999;5:85-90. [Crossref] [PubMed]
- Ernst A, Simoff MJ, Mathur PN, et al. D-light autofluorescence in the detection of premalignant airway changes: a multicenter trial. J Bronchol 2005;12:133-8. [Crossref]
- Kakihana M, Il KK, Okunaka T, et al. Early detection of bronchial lesions using system of autofluorescence endoscopy (SAFE1000). Diagn Ther Endosc 1999;5:99-104. [Crossref] [PubMed]
- Yokomise HMD, Yanagihara KMD, Fukuse TMD, et al. Clinical experience with lung-imaging fluorescence endoscope (LIFE) in patients with lung cancer. J Bronchol 1997;4:205-8. [Crossref]
- Venmans BJ, Van Boxem TJ, Smit EF, et al. Results of two years expenience with fluorescence bronchoscopy in detection of preinvasive bronchial neoplasia. Diagn Ther Endosc 1999;5:77-84. [Crossref] [PubMed]
- Furukawa K, Ikeda N, Miura T, et al. Is autofluorescence bronchoscopy needed to diagnose early bronchogenic carcinoma? Pro: Autofluorescence bronchoscopy. J Bronchol 2003;10:64-9. [Crossref]
- Shibuya K, Fujisawa T, Hoshino H, et al. Fluorescence bronchoscopy in the detection of preinvasive bronchial lesions in patients with sputum cytology suspicious or positive for malignancy. Lung Cancer 2001;32:19-25. [Crossref] [PubMed]
- Chen W, Gao X, Tian Q, et al. A comparison of autofluorescence bronchoscopy and white light bronchoscopy in detection of lung cancer and preneoplastic lesions: A meta-analysis. Lung Cancer 2011;73:183-8. [Crossref] [PubMed]
- Chiyo M, Shibuya K, Hoshino H, et al. Effective detection of bronchial preinvasive lesions by a new autofluorescence imaging bronchovideoscope system. Lung Cancer 2005;48:307-13. [Crossref] [PubMed]
- He Q, Wang Q, Wu Q, et al. Value of autofluorescence imaging videobronchoscopy in detecting lung cancers and precancerous lesions: a review. Respir Care 2013;58:2150-9. [Crossref] [PubMed]
- Ueno K, Kusunoki Y, Imamura F, et al. Clinical experience with autofluorescence imaging system in patients with lung cancers and precancerous lesions. Respiration 2007;74:304-8. [Crossref] [PubMed]
- Li Y, Li X, Sui XZ, et al. Comparison of the autofluorescence bronchoscope and the white light bronchoscope in airway examination. Chin J Cancer 2010;29:1018-22. [Crossref] [PubMed]
- Zaric B, Canak V, Stojanovic G, et al. Autofluorescence videobronchoscopy (AFI) for the assessment of tumor extension in lung cancer. Technol Cancer Res Treat 2009;8:79-84. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Anantham D, et al. Narrow-band imaging bronchoscopy increases the specificity of bronchoscopic early lung cancer detection. J Thorac Oncol 2009;4:1060-5. [Crossref] [PubMed]
- Cetti EJ, Nicholson AG, Singh S, et al. An evaluation of a videobronchoscopy-based autofluorescence system in lung cancer. Eur Respir J 2010;35:1185-7. [Crossref] [PubMed]
- Zaric B, Becker HD, Perin B, et al. Autofluorescence imaging videobronchoscopy improves assessment of tumor margins and affects therapeutic strategy in central lung cancer. Jpn J Clin Oncol 2010;40:139-45. [Crossref] [PubMed]
- Ikeda N, Honda H, Hayashi A, et al. Early detection of bronchial lesions using newly developed videoendoscopy-based autofluorescence bronchoscopy. Lung Cancer 2006;52:21-7. [Crossref] [PubMed]
- Zheng X, Xiong H, Li Y, et al. Application of Quantitative Autofluorescence Bronchoscopy Image Analysis Method in Identifying Bronchopulmonary Cancer. Technol Cancer Res Treat 2017;16:482-7. [Crossref] [PubMed]
- Zhu LY, Xu YJ, Liang D, et al. The clinical value of autofluorescence bronchoscopy for the diagnosis of lung cancer. Zhonghua Jie He He Hu Xi Za Zhi 2012;35:419-22. [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. [Crossref] [PubMed]
- Sutedja TG, Codrington H, Risse EK, et al. Autofluorescence bronchoscopy improves staging of radiographically occult lung cancer and has an impact on therapeutic strategy. Chest 2001;120:1327-32. [Crossref] [PubMed]
- Gabrecht T, Radu A, Grosjean P, et al. Improvement of the specificity of cancer detection by autofluorescence imaging in the tracheo-bronchial tree using backscattered violet light. Photodiagnosis Photodyn Ther 2008;5:2-9. [Crossref] [PubMed]
- Lovisa B, Gabrecht T, Andrejevic S, et al. Improvement of the Contrast in Cancer Detection by Autofluorescence Bronchoscopy using a narrow spectral violet Excitation: A preliminary study. Biomed Signal Process Control 2007;2:234-8. [Crossref]
- Gabrecht T, Radu A, Zellweger M, et al. Autofluorescence bronchoscopy: clinical experience with an optimized system in head and neck cancer patients Med. Laser Appl 2007;22:185-92. [Crossref]


