
What’s the best modality for patient selection for predicting response to PD-1/PD-L1 inhibitors?
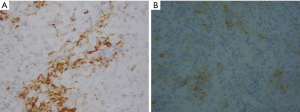
Lu S and colleagues made an exhaustive comparison of several biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade (1). The type of test impacts patient’s response to therapy and varies according to the country where it is performed. The FDA recommendation include the use of a specific anti-PD-L1 clone while EMA guidelines recommend the use of IHC validated test but this open the possibility to use different way of assay validation among the different laboratories i.e., companion diagnostic versus not companion diagnostic test. The use of the former has high costs and the adoption of an IHC platform depends on the instrument present in a center, and sometimes is company sponsored. In according to other authors, we found a different PD-L1 immunoreactivity on epithelial cells and macrophages by using different anti-PD-L1 antibody clones (SP142 vs. SP263, Roche Ventana, Tucson, AZ) (Figure 1). The lack of specific guidelines leads to discrepancies in technical and/or clinical validation procedures of PD-L1 testing. The authors underlined that IHC has some limitations due to the lack of reproducibility among the different antibody clones, platforms and cut offs used. No other tests are now included in the clinical practice because of the lack of comparison studies that included patients treated with immune checkpoint inhibitors selected by mIHC/IF, TMB or GEP. The main limitations of mIHC/IF are due to the unstandardized different selection of type of marker combination (epithelial alone or combination with intratumoral and/or peritumoral TME) that has been taken into consideration in the different studies, affecting their reproducibility. All the described biomarkers are characterized by dynamicity during the time and their cut offs are variable among the different tumor types, and this was not considered by the authors. The best biomarker combos should take in consideration multiple approaches given that all these biomarkers have different meaning and reflect inflammation and/or neoantigens productions. On the other hand these biomarkers are not evaluated on macrophages or cancer associated fibroblast that have an important role in anti-tumor immune response. Several pathologic and medical societies are making efforts for test harmonization to render them usable in the clinical practice (2,3). The optimal biomarker combos should have the maximum sensitivity and specificity but high sensitivity often means low specificity. The limits to overcome are lack of standardization of the tests, the costs and reimbursability of the methods. The harmonization of the national and international guidelines is still an urgency.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lu S, Stein JE, Rimm DL, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44-56. [Crossref] [PubMed]
- Marchetti A, Barberis M, Franco R, et al. Multicenter Comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) Assays to Test PD-L1 Expression for NSCLC Patients to Be Treated with Immune Checkpoint Inhibitors. J Thorac Oncol 2017;12:1654-63. [Crossref] [PubMed]


