
The prospect of combination therapy with immune checkpoint inhibitors and chemotherapy for squamous cell carcinoma of the lung
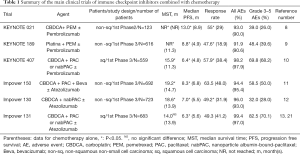
Non-small cell lung carcinoma (NSCLC) is the most common cause of cancer death in the world. Programmed cell death protein 1 (PD-1)-targeted therapies, which showed durable effects in patients with advanced NSCLC and superior survival benefits in comparison to chemotherapy in patients with advanced NSCLC, were established as breakthrough drugs by the development of immunotherapy (1-4). On the other hand, there is a population that is resistant to immune checkpoint inhibitors (ICI) because more than 30–40% of patients who receive ICI monotherapy develop progressive disease (PD) (5,6). We also reported that the PD was 43.2% in 44 patients with NSCLC who received anti-PD-1 antibody monotherapy. In this cohort, the Eastern Cooperative Oncology Group performance status (ECOG PS), pathological type, standardized uptake value (SUV) on positron emission tomography (PET), white blood cell count (WBC), neutrophil count, NLR (neutrophil-to-lymphocyte ratio), LDH (lactate dehydrogenase) and albumin were independent prognostic factors in patients receiving anti-PD-1 antibody monotherapy (7). Aggressive cancers, such as those with a high SUV on PET and a high LDH level, may not be sensitive to ICIs. To cover the ICI-resistant population, combination therapy with ICI and chemotherapy is administered to patients with NSCLC, and has shown a wide range of effects, as demonstrated in Table 1 (8-13). Combination therapy with ICI and chemotherapy significantly prolonged overall survival (OS) and progression free survival (PFS), and provided improvement of the objective response rate in almost all trials except for OS in IMpower 131. Although the programmed cell death protein ligand-1 (PD-L1) expression is an established biomarker that is used to predict the effect of anti-PD-1 antibody monotherapy (1,2,4), the clinical significance of the PD-L1 expression in patients receiving combination therapy with ICIs and chemotherapy remains unclear. Recently the PD-L1 expression is mainly confirmed for the indication of ICI monotherapy as second-line treatment or as a first line treatment in certain patients, such as those with chemotherapy-intolerable complications or PD-L1 high tumor cells. The effects of chemotherapy may cover ICI-resistant-populations such as PD-L1-low or -negative tumor cells or immune escape tumor cells. Additionally, the PD-L1 expression varies according to the tumor microenvironment. The change in the tumor microenvironment after the administration of chemotherapy is unpredictable. It is sometimes confusing to evaluate PD-L1 expression because of this heterogeneity. The effects of immunogenic cell death induced by anti-cancer drugs may enhance the antitumor immune response, the magnitude of the effects in the patient’s body is unclear. Moreover, the antitumor effect can be changed according to the dose of agents like methotrexate, which has immunosuppressive effect at high doses, but immunosupportive effect, by promoting the maturation of dendritic cells, at lower doses (14). In our multivariate analysis of 44 NSCLC patients treated with anti-PD-1 antibody monotherapy, the ECOG PS and albumin level before treatment were identified as independent prognostic factors (7). We think that early treatment with ICIs may be important for an effective immune responses before the appearance of cachexia.
Full table
Le et al. reported that a mean of 1,782 somatic mutations per tumor in mismatch repair—deficient tumors were detected by whole-exome sequencing, in comparison to 73 in mismatch repair—proficient tumors, and many somatic mutations brought better PFS in patients receiving anti-PD-1 antibody therapy (15). Somatic mutations, which are called “Neoantigens”, possess powerful immunogenicity. Theoretically, tumors with many somatic mutations related to mismatch-repair defects could be good candidates for immune check point inhibitors. CheckMate 227 showed that tumor mutation burden (TMB) was correlated with the clinical response to combination therapy with nivolumab and ipilimumab as first-line therapy for NSCLC. This trial was the first report to evaluate the TMB as a biomarker in NSCLC patients undergoing ICI therapy. Although a significant benefit such as prolonged PFS was not observed in the overall population of NSCLC patients who received combination therapy (nivolumab and ipilimumab) in comparison to chemotherapy alone, NSCLC patients with a TMB level of 10 ≥ mutation/Mbase who received combination therapy with nivolumab and ipilimumab showed significantly better PFS in comparison to those who received chemotherapy alone, irrespective of the PD-L1 expression (16). There is a possibility that the TMB is a novel biomarker for NSCLC patients receiving ICI therapy.
Squamous cell carcinoma (SCC) is highly associated with smoking, which accounts for 15–20% of NSCLC. ICIs therapy was associated with good effects in NSCLC patients with a smoking history. It is thought that smoking, which causes DNA damages due to the extremely toxic smoke from cigarettes, is strongly related with gene mutations of cancer cells. KEYNOTE 407 is a phase 3 clinical trial of carboplatin + paclitaxel or nanoparticle albumin-bound (nab)-paclitaxel ± pembrolizumab for SCC. Patients with SCC more frequently have current or previous smoking history compared to adenocarcinoma, which has a growing proportion composed of never-smokers or previous light smokers (17). SCC is affected by the genomic mutations derived from cigarettes carcinogenesis. TP53 mutations are the most frequent (>60–70%) genomic alteration found in SCC (18). It is thought that TP53 mutations might represent an immunogenic target for promoting an antitumor immune response, because we clarified that TP53 with a point mutation simultaneously induced both cellular and humoral immune responses in an NSCLC patient (19). Moreover, these TP53 mutation-specific cytotoxic T lymphocytes (CTL) and TP53-specific B lymphocytes accumulated in the tumor microenvironment. More than 5-year survival was observed without any other recurrences after adrenalectomy to treat postoperative single metastasis of the right adrenal gland. Thus, it is possible that the promotion of the immune response against TP53 mutation contributed to the good prognosis of the patient. Assoun et al. revealed that TP53 mutations brought a prognostic benefit in NSCLC patients treated with anti-PD-1 antibodies. The TP53 mutation status was independently associated with longer OS in a multivariate analysis [hazard ratio (HR): 0.35, 95% CI: 0.16–0.77, P=0.09) (20).
In KEYNOTE 407, 559 untreated-SCC patients underwent randomization. Two hundred seventy-eight patients were allocated to the pembrolizumab-combination group and 281 were assigned to the placebo-combination group. The median follow-up period was 7.8 months. The median OS was 15.9 months in the pembrolizumab-combination group and 11.3 months in the placebo-combination group (HR for death: 0.64%, 95% CI: 0.49–0.85, P<0.001). The median PFS was 6.6 months in the pembrolizumab-combination group and 4.8 months in the placebo-combination group (HR for progression or death: 0.56%, 95% CI: 0.45–0.70, P<0.001). The incidence of grade 3–5 adverse events (AEs) was 69.8% in the pembrolizumab-combination group and 68.2% in the placebo-combination group. The rate of discontinuation due to AEs was 13.3% in the pembrolizumab-combination group and 6.4% in the placebo-combination group. The incidence of pneumonitis of all grades and grade 3–5 was 6.5% and 2.5%, respectively, in the pembrolizumab-combination group and 2.1% and 1.1% in the placebo-combination group. Pneumonitis is the most lethal AE. Because SCC patients often have a smoking history, we should more pay attention to pneumonitis, which is also associated with smoking, as an AE. However, the usefulness of first line pembrolizumab-combination therapy was also confirmed in SCC. On the other hand, the OS benefit was independent of the PD-L1 expression level of the tumor cells. There was no significant difference in OS between the pembrolizumab-combination group and the placebo-combination group in the analysis of the subgroup of patients with PD-L1 >50% (10).
To evaluate the clinical outcomes of combination therapy with ICIs and chemotherapy in SCC patients, it is important to compare the results of KEYNOTE 407 and IMpower 131 (carboplatin + nab-paclitaxel with atezolizumab). IMpower 131 revealed that combination therapy using atezolizumab for SCC patients significantly improved PFS in comparison to chemotherapy alone, but not OS. Zhang et al. compared KEYNOTE 407 and IMpower 131 and revealed that pembrolizumab treatment was associated with significantly better OS (HR: 0.79, 95% CI: 0.47–0.94, P=0.02) and numerically superior PFS (HR: 0.79, 95% CI: 0.60–1.04, P=0.10) in comparison to atezolizumab in combination with chemotherapy, while they showed a similar ORR, and similar rates of all cause AEs and grade 3–5 AEs. In PD-L1-high SCC patients, pembrolizumab and atezolizumab demonstrated similar OS and PFS, while pembrolizumab showed significantly better OS (HR: 0.43, 95% CI: 0.24–0.76; P<0.01) and numerically superior PFS (HR: 0.83, 95% CI: 0.51–1.26, P<0.33) in comparison to atezolizumab in PD-L1-low SCC patients. Moreover, pembrolizumab demonstrated significantly better PFS (HR: 0.46, 95% CI: 0.28-0.75, P<0.01) in comparison to atezolizumab in patients with PD-L1-negative SCC (21). However, this analysis had a number of limitations. The follow-up periods were relatively short (the median follow-up periods in KEYNOTE 407 and IMpower 131 were 7.8 and 17.1 months, respectively). There was an issue with the concordance of anti-PD-L1 antibodies. The PD-L1 expression was evaluated using 22C3 in KEYNOTE 407 and SP142 in IMpower131. The sensitivity of SP142 is lower in comparison to 22C3 (22,23). Moreover, the SP142 immunohistochemistry (IHC) assay detects the PD-L1 expression of tumor cells and tumor-infiltrating immune cells, while the 22C3 assay only evaluates the PD-L1 expression in tumor cells. IMpower 131 has not yet been published and is only available as an American Society of Clinical Oncology (ASCO) meeting abstract. Therefore, the information of IMpower 131 was limited to those available in the ASCO meeting abstract (13,21). It is therefore difficult to compare these 2 trials directly.
Chen et al. analyzed the data from 11 clinical trials that included 3,112 SCC patients treated with monotherapy or combination therapy with ICIs or chemotherapy alone. ICI therapy including anti-PD-1 antibody and anti-PD-L1 antibody showed significant clinical advantage such as prolonged OS (HR: 0.74; P<0.001) and PFS (HR: 0.66; P<0.001) in SCC patients in comparison to chemotherapy. The clinical benefits of ICI therapy for SCC were similar in subgroup analyses that were performed according to the evaluation method of each clinical trial. However, no significant OS benefit was detected in SCC patients treated with anti-PD-L1 antibodies (HR: 0.87, P=0.087) (24). In IMpower 131, combination therapy with atezolizumab had no significant OS benefit in comparison to chemotherapy alone. In a subgroup analysis of the POPLAR trial, atezolizumab showed no significant benefit in terms of OS (HR: 0.80; 95% CI: 0.49–1.30) (25). However, Checkmate 017 showed that nivolumab brought significantly better PFS and OS in SCC patients (5). Moreover, combination therapy with pembrolizumab and chemotherapy showed significantly better PFS and OS in comparison to chemotherapy alone in KEYNOTE 407 (10). A meta-analysis using data from KEYNOTE 407 and IMpower 131 revealed that pembrolizumab brought significantly better OS (HR: 0.67, P=0.02) and numerically superior PFS (HR: 0.79, P=0.10) in comparison to atezolizumab in SCC patients (21). This difference might be due to the different ligands and signaling of each of molecules targeted by ICIs. Additionally, atezolizumab may not easily stimulate the immune microenvironment of SCC. A subgroup analysis according to the expression of PD-L1 showed that ICI therapy prolonged PFS and OS in SCC patients, regardless of the PD-L1 expression as shown in Checkmate 017 (5,23). Although SCC patients are a relatively homogenous population in comparison to non-squamous NSCLC patients, the clinical effects of ICIs are not associated with the PD-L1 expression in SCC patients. The reason for this is still unclear. Further fundamental research is therefore necessary to clarify the mechanism of the uncorrelated relationship between the clinical effect and the PD-L1 expression and to identify a novel biomarker for SCC. Considering the characteristics of SCC, such as the mutation load due to smoking, the TMB may be a better predictive biomarker, even though a high TMB was not associated with a significant PFS benefit in SCC patients undergoing combination therapy (nivolumab and ipilimumab) in comparison to those who received chemotherapy alone in CheckMate 227 (16). Additional studies are expected to confirm this. We think that it may be better to prioritize anti-PD-1 antibody over anti-PD-L1 antibody until new results of ICI therapy for SCC patients are obtained.
In conclusion, KEYNOTE407 revealed that combination therapy with pembrolizumab and chemotherapy brought significantly better OS and PFS in comparison to chemotherapy alone. IMpower 131 revealed that combination therapy using atezolizumab for SCC patients significantly improved PFS in comparison to chemotherapy alone, but not OS. In SCC patients, pembrolizumab may have a better OS benefit than atezolizumab in combination with chemotherapy. On the other hand, the incidence of grade 3–5 AEs was 69.8% in the pembrolizumab-combination group and 68.2% in the placebo-combination group. We should decide the treatment strategy taking into account individual patient risks and benefits based on patient characteristics.
Acknowledgments
Funding: Ichiki acknowledges support from Grant support: JSPS KAKENHI (18K08806, 19K09294).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.01.14). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Gettinger SN, Horn L, Gandhi I, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, bms-936558, ono-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 2015;33:2004-12. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Ichiki Y, Matsumiya H, Mori M, et al. Predictive factors of postoperative survival among patients with pulmonary neuroendocrine carcinoma. J Thorac Dis 2018;10:6912-20. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for adcanced, non-squamous non-small-cell lung cancer: a randomized, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small cell lung cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicenter, randomized, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36:LBA9000. [Crossref]
- Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012;11:215-33. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018;378:2093-104. [Crossref] [PubMed]
- Khuder SA, Mutgi AB. Effect of smoking cessation on major histologic types of lung cancer. Chest 2001;120:1577-83. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519-25. [Crossref] [PubMed]
- Ichiki Y, Takenoyama M, Mizukami M, et al. Simultaneously cellular and humoral immune response against mutated p53 in a patient with lung cancer. J Immunol 2004;172:4844-50. [Crossref] [PubMed]
- Assoun S, Theou-Anton N, Nguenang M, et al. Association of TP53 mutations with response and longer survival under immune check point inhibitors in advanced non-small-cell lung cancer. Lung Cancer 2019;132:65-71. [Crossref] [PubMed]
- Zhang Y, Zhou H, Li Zhang. Which is the optimal immunotherapy for advanced squamous non-small-cell lung cancer in combination with chemotherapy: anti-PD-1 or anti-PD-L1? J Immunother Cancer 2018;6:135. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blue print PD-L1 IHC assay comparison project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 2018;13:1302-11. [Crossref] [PubMed]
- Chen RL, Zhou JX, Cao Y, et al. The efficacy of PD-1/PD-L1 inhibitors in advanced squamous-cell lung cancer: a meta-analysis of 3112 patients. Immunotherapy 2019;11:1481-90. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicenter, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]


