
Lung cancer biomarker tests: the history and perspective in Japan
Introduction
The biomarker testing is recognized as being indispensable for selecting patients with advanced lung cancer (1). EGFR mutation is the first biomarker for therapeutic selection of lung cancer patients since the identification of the correlation between EGFR TKI response and EGFR mutation status. The second gene for target therapy was ALK fusions, then followed by ROS1 fusions, BRAF V600E mutation and NTRK fusions. These biomarker testing used various techniques including PCR for EGFR, FISH and IHC for ALK, reverse transcriptase PCR for ROS1, next-generation sequencing (NGS) for BRAF and NTRK. Concerning the increased number of analyzing genes and the limitation of tissue size, particularly in advanced-stage patients, multiplex panel testing has a great advantage in clinical practice, and indeed, the biomarker testing for genetic alterations is shifting to multiplex gene panel testing. Another essential therapeutic biomarker, PD-L1 IHC for immune checkpoint inhibitor (ICI), has a different concern regarding multiple assays with different clones (2). In this review, we introduce the history and current status of the biomarker testing for lung cancer in Japan and discuss perspectives, focusing on cell-free DNA (cfDNA)-based panel testing.
Current status of biomarker tests in Japan
Japanese public health care is similar to those of the UK, Canada and Australia. All people have to join a health insurance system, which covers most expenses of the individuals. Under this system, medical doctors request to use the medical procedures, equipment and drugs that have been approved by the Pharmaceuticals and Medical Devices Agency (PMDA) for reimbursement. The biomarker testing, including a series of companion diagnostics and in vitro diagnostics (IVD), is also included in the regulation. Currently approved biomarker tests for lung cancer treatment are listed in Table 1.
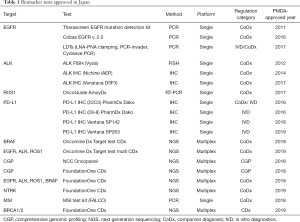
Full table
The Japan Lung Cancer Society (JLCS), a major academic organization on lung cancer, has been intensively involved in the promotion of research and distribution of knowledge regarding biomarker testing. The activities are exemplified by released five guidance for individual biomarker testing, some of which were released in English (3). The firstest one, the Guidance for EGFR Testing for Lung Cancer Patients, was published in 2009 and was revised four times according to the development of drugs and changes in companion diagnostics (currently, version 4.2 in March 2019). The latest one is the Guidance for multiplex gene testing using NGS, released in December 2019. The guidance recommends a simultaneous biomarker testing of the five targets (EGFR, ALK, ROS1, BRAF, PD-L1), using either multiple standalone or multiplex testing (Figure 1), which represents current biomarker testing recommended in Japan.
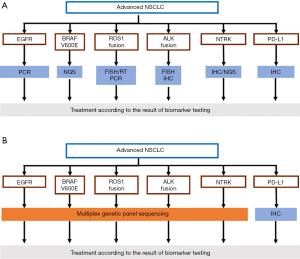
Standalone biomarker tests
Individual biomarker assays corresponding to particular targeted-agents have been developed, independently. Therefore, treating physicians used to use multiple standalone assays before the approval of multiplex gene testing. We first show historical changes and the current situation of such standalone assays for the five targeted genes.
EGFR
Because EGFR mutation was identified before the approval of EGFR tyrosine kinase inhibitor (EGFR-TKI), the assays of detecting EGFR mutations initially started with laboratory-developed tests (LDT). More than 90% of samples for molecular testing in Japan are examined in three large commercial companies, but they adapted different techniques, including PCR-invader, LNA-PNA clamping and Sanger sequencing, the last of which was replaced with cycleave PCR for higher sensitivity. This meant that the treating physicians had to select one of the LDTs, so a study was conducted to examine the concordance among the three assays (4), resulting in almost equal sensitivity and specificity across these assays. Later, Cobas EGFR® and Therascreen EGFR® were approved as a companion diagnosis for gefitinib and erlotinib, respectively, and, thereafter, these assays were used widely. All LDT assays had no regulation for cytology samples, while the companion assays are validated only for tissue specimens. However, most treating physicians do not know the difference in the companion diagnostics, and ignores the limitation. After approval of osimertinib for resistant T790M mutation, Cobas EGFR version 2 was approved for either cell-free DNA (cfDNA) in the plasma and tissue specimens. A blood sample is easy to access, but cfDNA testing is restricted to the patients, any of whom tissue specimens cannot be obtained.
ALK
Currently, several assays including FISH, IHC and NGS, have been approved for detecting ALK fusions. Similar to the US, the Vysis® ALK Break Apart FISH probe kit is the first companion diagnosis for crizotinib. As approval of the agent was so rapid, most laboratories could not prepare to accept lung cancer samples for FISH analysis in a large batch, even though the routine ALK testing was recommended. Together with promoting an efficient screening program, the JLCS released the recommendation to use ALK IHC for screening, followed by FISH confirmation if IHC is positive. In 2014, the Nichirei ALK iAEP IHC kit, using the clone 5A4, was approved for alectinib, but even if the ALK IHC was positive, FISH confirmation was requested to start the treatment with alectinib according to the regulation of the PMDA. Subsequently, the Ventana ALK IHC (D5F3) was approved as a companion diagnostic test for crizotinib, and this IHC test did not need FISH confirmation if positive. Therefore, many treating physicians worried about not having reimbursement when the Nichirei ALK IHC was used for crizotinib because the Nichirei ALK IHC was the companion diagnostic test for alectinib. In response to such a situation, the JCLS made a statement in 2014 that the diagnostic kits should correspond to driver alternations but not drugs. This statement relieved the treating physicians and the IHC vendors expanded to the designation to other ALK agents by submitting the concordance data to the regulatory authorities. Currently, either IHC assay is used regardless of the selected ALK inhibitors.
PD-L1
Nivolumab is the first approved immune checkpoint inhibitor (ICI) in Japan, and the approval of such an expensive drug caused a general concern that its broad use may result in a huge burden for the budget of the national health care system. Initially, the treatment of a patient cost about 320,000 USD a year, and all expenses were covered by the public health care program. Using a particular regulation rule, the government currently lists the drug price that is now 23.8% of the initial one. Somehow associated with public concerns, PD-L1 IHC was not requested in the beginning to treat a patient with nivolumab. However, at the time of pembrolizumab approval, nivolumab treatment was restricted to patients diagnosed with adenocarcinoma and a TPS ≥1%. For squamous cell carcinoma, PD-L1 IHC is not requested for nivolumab treatment. Of note, the regulation authorities permitted to adapt the TPS of PD-L1 IHC 22 C3 pharmDx, which was a companion diagnostic test of pembrolizumab, instead of PD-L1 28-8 IHC developed for nivolumab. During this period, pembrolizumab was the sole agent eapplied in the first line, so most patients with advanced NSCLC were examined with the PD-L1 22C3 IHC. This regulation rule of PD-L1 IHC was applied to subsequent ICIs despite multiple PD-L1 assays having been developed for each ICI. Currently, PD-L1 IHC is requested for the atezolizumab treatment for squamous cell carcinoma and for durvalumab as post-chemoradiotherapy in stage III patients, and these treatments are reimbursed to the patients with a TPS ≥1% with PD-L1 22C3 IHC, not to those with PD-L1 SP142 and SP263 assays, corresponding to atezolizumab and durvalumab, respectively. This implies that Japan was the first country that adapted several harmonization studies to integrate multiple PD-L1 assays into a representative one. In line with this regulation, Japan does not have a category of complementary diagnosis in contrast to the US.
ROS1
OncoGuide AmoyDx ROS1 fusion gene detection kit, which is a ROS1 detection system based on real-time RT-PCR, is a sole companion diagnostic test before the approval of Oncomine Dx target test (Oncomine Dx TT, described later). This assay can accept either FFPE fresh frozen tumor tissue or cytology samples including pleural fluid and bronchial washing, but FFPE specimens were a major source of this analysis due to difficulty of handling of frozen materials in clinical practice. As expected, the success rate of this assay limited to about 70–80%. Together with a low incidence of ROS1 fusions in NSCLCs, a current testing rate of ROS1 testing was limited to be low. According to the meeting report by Nishino et al., a multicenter retrospective study involving major Japanese cancer center showed that ROS1 testing was conducted in only 67% of the patients, who were treated with some kinds of drugs during Aug 2017 to Dec 2017 (Table 2) (5). The testing rate was highly contrasted with those of EGFR (97.5%), ALK (88.1%) and PD-L1 IHC (87.1%). The low testing rate has been greatly improved with the approval of a multiplex genetic test.
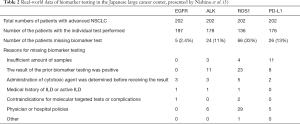
Full table
BRAF
Similar to the US, Oncomine Dx TT was a first companion diagnostic test using an NGS technique. This NGS test can analyze 46 genes and 21 fusions, but only BRAF V600E status was initially reported to the clinic because the NGS test was approved just for BRAF V600E. After the approval of other driver genes, the treating physicians can access all information obtained with this technique. Cobas BRAF V600 assay has been also approved for melanoma patients, but not for lung cancer patients. Therefore, if a sample was failed with the Oncomine Dx TT test, there are no standalone tests to access BRAF V600 status under the current national healthcare reimbursement program.
Multiplex cancer gene panel tests
In June 2019, the PMDA approved three NGS assays; the Oncomine Dx TT, the FoundationOne CDx (F1CDx) and the OncoGuide NCC OncoPanel (NCC Oncopanel). The characteristics were summarized in Table 3. The first two are familiar worldwide, but the NCC-OP is unique to Japan. The NCC Oncopanel was a comprehensive genomic profiling test, developed by a collaboration of the National Cancer Center and Sysmex Corporation (6). The test covers 114 genes and 12 fusions, characterized by a simultaneous sampling of blood, which is utilized to address accurate somatic and germline mutations in addition to tumor mutation burden. The report of the first 230 cases analyzed with this panel (7) was similar to those of the other panels in terms of ratio to lead to the CGP-guided treatment (8,9).

Full table
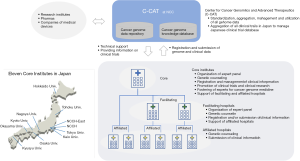
There are three approved panel tests, but not all hospitals in Japan can conduct these tests with the national healthcare reimbursement coverage. The PMDA categorized panel tests into two groups, a conventional IVD and a comprehensive genomic panel (CGP) test, based on the number of genes and necessity of genetic consultation for germline mutations. The F1CDx and NCC Oncopanel are of the CGP test because the panels examine more than 100 genes and have a possibility to suggest or detect germline mutations. For the CGP testing, the Japanese Ministry of Health, Labor, and Welfare (MHLW) organizes a nation-wide network for cancer genome medicine, in which 11 core institutes, 34 facilitating and 122 affiliated hospitals were designated (Figure 2). CGP testing is limited to these hospitals, but each designation has different roles as will be discussed later. Furthermore, this program is designed for patients with solid tumors that progress after standard therapy and/or rare types of cancer, such as pediatric cancers and sarcomas. The analyzing cost is reimbursed by the national healthcare reimbursement program only when an expert panel discussion is held. The expert panel, which functions like the molecular cancer board in the US, is strongly emphasized in this program, and its active operation with a multidisciplinary team is requested. In addition, the expert panel can be held only in the 11 core and 23 facilitating institutes, so the treating physicians in the affiliated hospitals have to attend the expert panel held at the core or facilitating institutes. This inconvenience is associated with national policy on the cancer genome medicine program. All genome data with clinical information should be submitted to the Center for Cancer Genomics and Advanced Therapeutics (C-CAT) (10), which was established by the Japanese government within the National Cancer Center in June 2018. The C-CAT functions as a hub for aggregating and managing nationwide information on cancer genomic medicine, as well as utilizing this data to enhance the quality of treatment and developing new treatments in collaboration with research facilities and pharma in Japan and worldwide. To establish a special connection to the C-CAT, the facilities were restricted to the 11 core institutes and 51 facilitating hospitals, which cover all over Japan.
Perspectives of biomarker testing in Japan
At the beginning of 2020, panel testing based on cfDNA has not been approved in Japan. As stated, cfDNA-based Cobas EGFR v.2 was commonly used to detect resistant T790M EGFR mutation, whereas a shift to the first line osimertinib is changing the situation. Some institutes conduct the Gardant 360 outside the reimbursement program, mostly under clinical trials, but liquid-based panel testing is under development. In 2019, the SAKIGAKE destination, equivalent to breakthrough therapy of the FDA, includes some anti-cancer agents, which are accompanied by cfDNA-based companion diagnostics. Therefore, panel testing using cfDNA will be approved in 2020 mostly within the category of the IVD. In terms of the cfDNA-based CGP testing, the MHLW and PMDA have an idea of a strong distinction between IVD and CGP, the approval may still need some more time.
Conclusions
Biomarker testing in Japan largely follows those corresponding to the US. However, there are some differences due to the national health care program and the medical environment. We reviewed the history of individual biomarker testing with some perspective in Japan.
Acknowledgments
This work was supported by the Research Promotion Grant of NCCI (Tokyo, Japan).
Fungding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.09). The series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” was commissioned by the editorial office without any sponsorship or funding. NM reports personal fees from AstraZeneca, Chugai Pharma, Miraca Life Sciences, MSD, Agilent, Novartis, Taiho, and Roche Diagnostics; institutional research funding from Roche Diagnostics and NEC, outside of this work. YY reports personal fees from AstraZeneca, Chugai Pharma, Pfizer, MSD, Agilent/Dako, Novartis, Roche Diagnostics, Sysmex, Daiichi-Sankyo, Amgen and Bristol-Myers/Ono.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. J Thorac Oncol 2018;13:323-58. [Crossref] [PubMed]
- Kerr KM, Tsao MS, Nicholson AG, et al. Programmed Death-Ligand 1 Immunohistochemistry in Lung Cancer: In what state is this art? J Thorac Oncol 2015;10:985-9. [Crossref] [PubMed]
- Biomarker Committee tJLCS. Guidance for ALK Gene Testing in Lung Cancer Patients. Available online: https://www.haigan.gr.jp/uploads/files/photos/641.pdf
- Goto K, Satouchi M, Ishii G, et al. An evaluation study of EGFR mutation tests utilized for non-small-cell lung cancer in the diagnostic setting. Ann Oncol 2012;23:2914-9. [Crossref] [PubMed]
- Nishino K, Masago K, Kurata A, et al. Real-world data of biomarker testing before first line treatments for the patients with advanced NSCLC- Multi-center retrospective study. Tokyo, Haigan: Annual meeting of the Japanese Lung Cancer Society, 2018;58:585.
- Center NC. Cancer Gene Panel Test OncoGuideTM NCC Oncopanel System added to Health Insurance Coverage list. Available online: https://www.ncc.go.jp/en/information/press_release/20190717/20190717152024.html
- Sunami K, Ichikawa H, Kubo T, et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci 2019;110:1480-90. [Crossref] [PubMed]
- Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016;1:e87062. [Crossref] [PubMed]
- Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 2017;23:703-713. [Crossref] [PubMed]
- The Center for Cancer Genomics and Advanced Therapeutics (C-CAT). Available online: https://www.ncc.go.jp/en/c_cat/


