Open-label, multicenter, randomized phase II study on docetaxel plus bevacizumab or pemetrexed plus bevacizumab for treatment of elderly (aged ≥75 years) patients with previously untreated advanced non-squamous non-small cell lung cancer: TORG1323
Introduction
Of all cancers worldwide, lung cancer has the highest incidence and is the leading cause of death (1). Almost half of patients with lung cancer are diagnosed at ≥75 years of age in Japan (2). The number of lung cancer patients, especially elderly patients, is increasing every year. Development of treatment strategies against non-small cell lung cancer (NSCLC) in this population is urgently required. Some guidelines state that treatment does not need to differ according to age if the patient has a good performance status (PS) (3,4). However, docetaxel monotherapy has been reported to have good efficacy for advanced NSCLC in elderly patients (5). Moreover, addition of cisplatin failed to show any survival benefit in the Japanese population (6). Docetaxel monotherapy is still the most widely used treatment for NSCLC in Japan
Bevacizumab is an anti-vascular endothelial growth factor monoclonal antibody. Addition of bevacizumab to carboplatin plus paclitaxel therapy for advanced non-squamous NSCLC has been shown to significantly prolong overall survival (OS), and decrease mortality by 21%. Subgroup analysis showed that the hazard ratio (HR) for OS in patients aged ≥70 years was not significant; multivariate analysis showed that only male sex, adrenal involvement, and carboplatin plus paclitaxel combination, and not old age, were independent poor prognostic factors. A retrospective cohort study also reported that the addition of bevacizumab prolonged OS among elderly patients (7). In contrast, another retrospective cohort study and combined analysis of the ECOG4599 and POINTBREAK trials failed to prove any survival benefit (8,9). Therefore, a prospective study is needed to determine whether combining bevacizumab with a cytotoxic agent yields survival benefits in elderly patients with non-squamous NSCLC.
Limited data are available on the effectiveness of bevacizumab plus a single cytotoxic agent against chemo-naïve non-squamous NSCLC. A prospective cohort trial conducted in Europe and Asia (SAiL study) analyzed and compared the efficacies of different combination regimens. Only 42 (1.9%) of 2,212 patients received a combination of monotherapy and bevacizumab. However, these data were encouraging; median time-to-progression and OS were 7.0 and 9.4 months, respectively (10).
We previously reported the feasibility of the combination of a single agent, docetaxel (TORG1014 study) or pemetrexed (TORG1015 study), and bevacizumab in elderly patients with NSCLC and provided promising preliminary data of the efficacy of these combinations (11,12).
The efficacy of a single cytotoxic agent plus bevacizumab has not been examined prospectively in chemo-naïve elderly patients with non-squamous NSCLC. Herein, we conducted an open-label multicenter randomized phase II study of docetaxel or pemetrexed plus bevacizumab in previously untreated elderly patients (≥75 years) with advanced non-squamous NSCLC. In this study, we aimed to assess the efficacy of these combinations and to choose the optimal regimen for elderly patients with non-squamous NSCLC.
Methods
Study design
This study was an open-label multicenter randomized phase II study of docetaxel or pemetrexed plus bevacizumab in elderly (≥75 years) patients with previously untreated advanced non-squamous NSCLC. This study was approved by the Institutional Review Board of all participating hospitals. This trial is registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR). The identification number is UMIN000012786. Enrollment was started in February 2014.
Patient eligibility
Eligible patients had the following characteristics: histologically or cytologically confirmed non-squamous NSCLC; Eastern Cooperative Oncology Group (ECOG) PS of 0 or 1; measurable lesions as defined by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1; no history of chemotherapy [except uracil and tegafur as adjuvant chemotherapy, and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor or anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitor in patients proven to harbor the activating EGFR mutation or ALK translocation]; ineligibility for receiving cisplatin bolus or combination chemotherapy; adequate bone marrow reserve; unimpaired hepatic and renal functions {leucocyte count ≥4,000/mm3; absolute neutrophil count ≥2,000/mm3; platelet count ≥100,000/mm3; hemoglobin level ≥9.5 g/dL; serum aspartate aminotransferase ≤2.5× upper limit of normal (ULN) range; alanine aminotransferase level ≤2.5× ULN range; total bilirubin ≤1.5 mg/dL; serum creatinine ≤1.5 mg/dL, and urinalysis of proteinuria examined by dipstick [dipstick result: negative, trace (+/−), or 1+]}. The key exclusion criteria were as follows: presence of symptomatic brain metastasis; history of hemoptysis (≥2.5 mL); active infectious disease; massive pleural, pericardial, or abdominal effusion; severe co-morbid diseases (heart disease, interstitial lung disease, inadequately controlled hypertension, poorly controlled diabetes mellitus); requirement of regular insulin injections; history of thoracic irradiation; concomitant malignancy within the last 5 years; coagulation disorders or therapeutic use of anti-coagulants; gastrointestinal perforation; minor surgery in the past 2 weeks; and major surgery in the last 4 weeks, or major surgery with lobectomy/pneumonectomy in the last 8 weeks.
Trial design and treatment
This was an open-label trial. Patients were randomized 1:1 to receive either docetaxel, or pemetrexed with bevacizumab. Bevacizumab (15 mg/kg) was administered to patients in both arms on day 1, every 3 weeks. Docetaxel (50 mg/m2) was administered in the docetaxel plus bevacizumab arm on day 1, every 3 weeks; pemetrexed (500 mg/m2) was administered in the pemetrexed plus bevacizumab arm on day 1, every 3 weeks; these agents were administered until disease progression or development of unacceptable toxicity (11,12). Patients treated with pemetrexed plus bevacizumab received folic acid and vitamin B12 as premedication, according to the pemetrexed package insert.
Assessment
Tumor imaging was performed every 6 weeks in the first 6 months and every 9 weeks thereafter. Treatment response was assessed according to RECIST version 1.1. Objective response rate (ORR) and progression-free survival (PFS) were evaluated by the investigators and an independent radiologist. Adverse events and laboratory abnormalities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03. Quality of life (QoL), using the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire, was assessed before treatment, at weeks 6, 12, 18, and 24, and at the end of treatment. EGFR mutation status was assessed using commercially available highly-sensitive methods such as the peptide nucleic acid-locked nucleic acid polymerase chain reaction (PCR)-clamp, cycleave PCR, PCR-Invader, Scorpion ARMS, or Cobas (Roche, Basel, Switzerland) methods in commercial laboratories or research institutions in Japan. ALK rearrangement was assessed by performing immunohistochemistry, fluorescence in situ hybridization, or reverse transcription PCR with tumor samples.
Statistical analysis and study endpoints
Efficacy was analyzed in the intention-to-treatment population, which included patients who fulfilled the eligibility criteria. Safety analysis was performed for all randomized patients who received at least one dose of assigned therapy. The Kaplan-Meier method was used for estimating OS, and PFS. Patients who were alive or lost to follow-up were censored at the time of data cut-off in OS analysis. Patients who were alive and did not have disease progression were censored at the end of the last imaging follow-up in PFS analysis. The log-rank method was used for assessing the differences in PFS and OS between the two groups. The Fisher’s exact test was used to assess the differences in ORR and adverse events between the two groups. The Student’s t-test was used to compare QoL, assessed as a change in scores compared to baseline. The primary endpoint was PFS by independent review. Secondary endpoints were safety, PFS by investigators, ORR, OS, and QoL. Selection design was applied in this study. Pemetrexed plus bevacizumab is generally less toxic and more feasible than docetaxel plus bevacizumab. We determined that the pemetrexed plus bevacizumab combination would be better for further evaluation if the true HR, and point estimate were 1.0, and ≤1.20, respectively. Contrarily, the docetaxel plus bevacizumab combination would be chosen if the true HR and point estimate were 1.42, and >1.20, respectively. The hypothesis would be accepted with ≥80% accuracy on occurrence of a total of 94 progression-free events. We assumed that the median PFS was 5.5 months. The planned sample size was 120 patients. Analyses were conducted using the SAS 9.4 statistical software (SAS Institute Inc., Cary, NC, USA).
Results
Patients characteristics
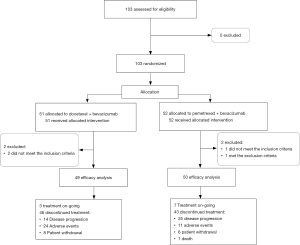
This study duration was from April 10, 2014, to May 11, 2017; however, enrollment was terminated early because of slow accrual. A total of 103 patients from 45 institutions were randomly assigned to the docetaxel plus bevacizumab (51 patients) and pemetrexed plus bevacizumab (52 patients) arms (Figure 1). All patients received one or more cycles of treatment. Four patients were finally excluded from efficacy analysis because 3 patients did not meet the inclusion criteria (all had low creatinine clearance [<45 mL/min]) and one met the exclusion criteria (had comorbid diabetes mellitus and required regular insulin injections). A total of 99 patients (49, docetaxel plus bevacizumab arm; 50, pemetrexed plus bevacizumab arm) were included in the full analysis set (FAS).
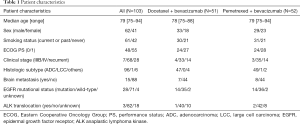
Table 1 summarizes patient characteristics in each arm. Patient and disease characteristics were well balanced between the two arms. The median age was 79 years (range, 75–94 years), and 62 (60.2%) patients were male. Of the patients, 61 (59.2%) had smoking history, 48 (46.6%) had PS of 0, 96 (93.2%) had adenocarcinoma histology, and 15 (14.6%) had asymptomatic or previously treated brain metastases. EGFR mutation and ALK translocation were detected in 28 (27.2%) and 3 (2.9%) patients, respectively. Twenty-five (24.3%) patients (12, docetaxel plus bevacizumab; 13, pemetrexed plus bevacizumab) received EGFR tyrosine kinase inhibitor treatment before enrollment. No patients received ALK tyrosine kinase inhibitor treatment before enrollment.
Full table
The median number of administered docetaxel and pemetrexed cycles were 5, and 7.5 cycles in FAS, respectively (P=0.129) (Table S1). The relative dose intensities of docetaxel and pemetrexed were 82.0% (range, 62–100%) and 94.0% (range, 76–100%), respectively. A significantly higher number of patients required dose reduction in the docetaxel arm (32 patients, 65.3%) than in the pemetrexed arm (9 patients, 18.0%) (P<0.001). The median number of administered bevacizumab cycles were 5 cycles in the docetaxel arm, and 6 cycles in the pemetrexed arm (P=0.311). The relative dose intensities of bevacizumab were 98.0% (range, 66–100%) and 96.5% (range, 56–100%) in the docetaxel and pemetrexed arms.

Full table
Efficacy
PFS analysis
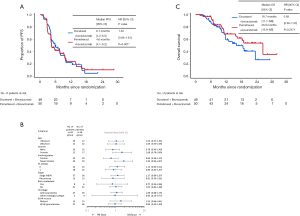
At the data cut-off, median follow-up duration was 11.2 months (range, 1.7–32.2 months). Of the total patients in both arms, 93 discontinued the protocol treatment. According to central assessment, a total of 77 progression-free events occurred in both arms. Median PFS values were 6.1 months [95% confidence interval (CI), 4.3–8.0 months] and 4.6 months (95% CI, 4.1–9.2 months) in the docetaxel plus bevacizumab and pemetrexed plus bevacizumab arms, respectively (HR, 1.03; 95% CI, 0.66–1.61) (Figure 2A). The 6-, and 12-month PFS rates were 50.6% (95% CI, 35.8–63.6%) and 23.1% (95% CI, 11.3–37.4%) in the docetaxel plus bevacizumab arm and 43.9% (95% CI, 29.6–57.3%) and 24.9% (95% CI, 13.0–38.8%) in the pemetrexed plus bevacizumab arm, respectively. Subgroup analysis showed that the HRs of PFS in female patients, never smokers, and EGFR mutation-positive patients exceeded 1.20 (Figure 2B). Median PFS values assessed by investigators were 7.6 months (95% CI, 5.7–9.8 months) and 7.5 months (95% CI, 4.3–9.6 months) in the docetaxel plus bevacizumab and pemetrexed plus bevacizumab arms, respectively (HR, 1.06; 95% CI, 0.66–1.68).
Objective response
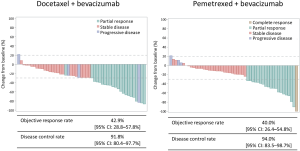
Figure S1 shows the waterfall plots, ORRs, and disease control rates (DCRs) in each arm as assessed by the independent review committee. The ORRs in the docetaxel plus bevacizumab and pemetrexed plus bevacizumab arms were 42.9% (95% CI, 28.8–57.8%) and 40.0% (95% CI, 26.4–54.8%), respectively (P=0.839). The DCRs in the docetaxel plus bevacizumab and pemetrexed plus bevacizumab arm were 91.8% (95% CI, 80.4–97.7%) and 94.0% (95% CI, 83.5–98.7%), respectively (P=0.715). One patient (2.0%) achieved complete response in pemetrexed plus bevacizumab arm.
Survival analysis
A total of 39 events occurred at the data cut-off. Median OS values in the docetaxel plus bevacizumab and pemetrexed plus bevacizumab arms were 18.7 months [95% CI, 11.7–not reached (NR)] and 26.6 (95% CI, 15.9–NR), respectively (Figure 2C). The HR was 0.69 (95% CI, 0.36–1.30; P=0.247). The 1-year OS rates were 60.5% (95% CI, 43.3–73.9%) and 69.7% (95% CI, 52.4–81.7%) in the docetaxel plus bevacizumab and pemetrexed plus bevacizumab arms, respectively.
Safety
All 103 randomized patients received at least one chemotherapy dose and were included in safety analysis. Grade ≥3 adverse events occurred in 47 (92.2%) of the 51 patients in the docetaxel plus bevacizumab arm and 39 (75.0%) of the 52 patients in the pemetrexed plus bevacizumab arm (Table 2). The most common grade ≥3 adverse events were leukopenia, neutropenia, febrile neutropenia, hypertension, and fatigue. The frequency of leukopenia (P<0.001), neutropenia (P<0.001), and fatigue (P=0.027) were significantly higher in the docetaxel plus bevacizumab arm than in the pemetrexed plus bevacizumab arm. The incidence of any-grade adverse events, such as leukopenia (P<0.001), neutropenia (P<0.001), and anemia (P=0.003), were significantly high in the docetaxel plus bevacizumab arm. Contrastingly, the incidence of any-grade thrombocytopenia was significantly high in the pemetrexed plus bevacizumab arm (P=0.002). Of the 51 patients in the docetaxel plus bevacizumab arm, 35 (68.6%) received significantly high amounts of granulocyte colony-stimulating factor for prophylaxis or treatment of neutropenia (P<0.001). Adverse events of special interest (AESI), including hypertension, proteinuria, wound healing complications, gastrointestinal perforations, arterial and venous thromboembolic events, hemoptysis, central nervous system bleeding, other hemorrhages, and congestive heart failure, occurred in 45 (88.2%) patients in the docetaxel plus bevacizumab arm and in 42 (80.8%) patients in the pemetrexed plus bevacizumab arm. Grade ≥3 AESIs occurred in 10 (19.6%) patients in the docetaxel plus bevacizumab arm and in 14 (26.9%) patients in the pemetrexed plus bevacizumab arm. Grade 5 adverse events, such as abdominal aortic aneurysm rupture, occurred in one patient in the pemetrexed plus bevacizumab arm.
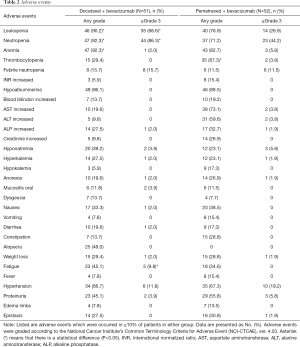
Full table
QoL analysis
In total, 32 (62.7%), and 36 patients (69.2%) in the docetaxel plus bevacizumab, and pemetrexed plus bevacizumab arms, respectively, submitted questionnaires before and after treatment. The mean baseline values of each score were similar between the two arms, except for the score for functional well-being, which was 19.1 (95% CI, 17.4–20.8) in the docetaxel plus bevacizumab arm and 16.1 (95% CI, 14.1–18.0) in the pemetrexed plus bevacizumab arm (P=0.022). With the exception of the scores for emotional well-being, these scores decreased after treatment in both arms. Mean change in the lung cancer subscale scores compared to baseline was significantly lower in the pemetrexed plus bevacizumab arm than in the docetaxel plus bevacizumab arm (−3.8 vs. −1.0; P=0.016) (Table 3). After 12 weeks, the changes in FACT-L total scores, Trial Outcome Index (TOI) scores, and physical well-being subscale scores were also significantly lower in the pemetrexed plus bevacizumab arm than in the docetaxel plus bevacizumab arm.
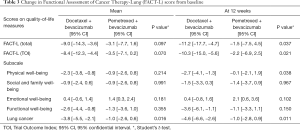
Full table
Discussion
The results of this randomized phase II study of docetaxel or pemetrexed plus bevacizumab in elderly patients with previously untreated advanced non-squamous NSCLC showed that both treatments had similar efficacies. The HR of PFS was 1.03; this was less than the predefined margin of 1.20 required for choosing pemetrexed plus bevacizumab treatment. A good toxicity profile was observed in the pemetrexed plus bevacizumab arm in terms of grade ≥3 hematological toxicities and fatigue, although one grade 5 bevacizumab-related adverse event occurred in the pemetrexed plus bevacizumab arm. Moreover, QoL analysis showed less deterioration in the pemetrexed plus bevacizumab arm. Therefore, the combination of pemetrexed and bevacizumab would be a candidate for further evaluation.
Traditionally, vinorelbine or gemcitabine monotherapy has been reported to prolong OS in elderly patients with advanced NSCLC (13,14). A Japanese phase III trial achieved a median OS and PFS of 14.8 months (95% CI, 11.9–24.1 months) and 4.4 months (95% CI, 3.4–5.1 months), respectively, with docetaxel monotherapy (6). Contrastingly, two single-arm phase II trials of pemetrexed monotherapy in elderly patients with non-squamous NSCLC failed to show its efficacy (15,16). Docetaxel monotherapy is still considered as the standard of care for elderly patients with non-squamous NSCLC in Japan.
Some studies have been conducted on the efficacy of platinum doublets plus bevacizumab in elderly patients with non-squamous NSCLC. The randomized phase II trial, 65Plus, that aimed to prove the efficacy of the addition of carboplatin to pemetrexed and bevacizumab as first-line treatment in elderly (≥65 years) patients with non-squamous NSCLC failed to show the non-inferiority of pemetrexed plus bevacizumab against carboplatin and pemetrexed plus bevacizumab (17). However, PFS in patients aged ≥70 years was comparable between the pemetrexed plus bevacizumab, and carboplatin with pemetrexed plus bevacizumab arms. Another study reported similar promising results against the combination of carboplatin, pemetrexed, and bevacizumab as first-line treatment in elderly (aged ≥75 years) patients with non-squamous NSCLC, with an ORR of 58%, and median PFS of 8.4 months (95% CI, 4.4–10.5 months) (18). This study was terminated early due to slow accrual. The clear reason for these results was unknown; however, we speculate that the indication of bevacizumab with platinum doublet therapy might be limited to a very small population of elderly patients with non-squamous NSCLC. Thus, addition of bevacizumab to platinum doublet therapy is still controversial in elderly patients with non-squamous NSCLC.
No clear evidence is available regarding whether addition of bevacizumab to monotherapy enhances anticancer activity. A randomized phase II trial including previously treated patients with NSCLC reported a median PFS of 4.8 months with bevacizumab plus docetaxel or pemetrexed, and 3.0 months with chemotherapy alone (19). A randomized phase II study including chemo-naïve non-squamous patients with NSCLC with PS-2 reported promising results, with an ORR of 31%, and median PFS of 4.0 months with pemetrexed plus bevacizumab (20). Compared to the response rate with pemetrexed alone (15%), a more than 2-fold increase in response rate was observed. Our previous studies including elderly patients with non-squamous NSCLC also showed promising results (11,12). The ORRs and median PFS were 28.6% (95% CI, 11.3–52.2%) and 5.9 months (95% CI, 3.6–9.1 months) in docetaxel plus bevacizumab combination, and 25.0% (95% CI, 5.5–57.2%), and 5.4 months (95% CI, 1.1–8.8 months), respectively, in the pemetrexed plus bevacizumab combination. In this TORG1323 study, we achieved ORRs and PFS of 42.9% and 6.1 months in the docetaxel arm and of 40.0% and 4.6 months in the pemetrexed arm, respectively. These results indicate that the addition of bevacizumab to monotherapy enhances its efficacy and should be encouraged.
Generally, the administration of 60 mg/m2 docetaxel monotherapy every 3 weeks is prevalent in Japan. The docetaxel dosage in this trial was decreased to 50 mg/m2 based on the previous study (11). In the JCOG0803/WJOG4307L, the incidence rates of grade ≥3 neutropenia and febrile neutropenia were reported to be 88.8%, and 15.2%, respectively, in elderly chemo-naïve patients receiving docetaxel monotherapy. Contrastingly, in this study, the incidence rates of grade ≥3 neutropenia and febrile neutropenia were 86.2% and 15.7%, respectively in patients receiving docetaxel plus bevacizumab, and were 44.2% and 11.5%, respectively in those receiving pemetrexed plus bevacizumab. Therefore, these combinations are considered to be tolerable. However, the protocol treatment was terminated before disease progression in 24 (49.0%) of 49 patients receiving docetaxel plus bevacizumab, and 12 (24.0%) of 50 patients receiving pemetrexed plus bevacizumab. These factors need to be considered while using these treatments in practice. Grade ≥3 AESIs occurred in 29% patients receiving monotherapy plus bevacizumab in the SAiL study (10). In this study, grade ≥3 AESIs occurred in 10 (19.6%) and 14 (26.9%) patients receiving docetaxel plus bevacizumab, and pemetrexed plus bevacizumab, respectively. Our study confirmed the safety of the addition of bevacizumab to monotherapy in elderly patients with non-squamous NSCLC.
Although the combination of pemetrexed and bevacizumab was found to be a promising treatment option in elderly patients with non-squamous NSCLC, this study has several limitations. First, this study was terminated early because of slow accrual, and the number of progression-free events was less than previously planned. However, a total 77 progression-free events finally occurred. This warranted accuracy rates of 78.8%, and 77.7% for correctly estimating the true efficacies of pemetrexed plus bevacizumab, and docetaxel plus bevacizumab, respectively. The aforementioned phase II study of carboplatin, pemetrexed, and bevacizumab in elderly patients with non-squamous NSCLC was terminated early because of slow accrual; only 12 patients were enrolled in >4 years. In this study, the number of enrollments was limited to 103 patients from 45 hospitals in >3 years, although 90 hospitals participated. The major reasons for slow accrual in bevacizumab-related studies in elderly patients might include poor PS, low creatinine clearance, eligibility for bevacizumab use (direct invasion of a bronchus or vessel, hemoptysis history, full-dose anticoagulant use, arterial/venous thromboembolism events, or chest irradiation), and history of other malignant diseases. Another limitation was that selection design was applied, which means that the current standard of care was not used as a comparator. A bias in patient-selection might have resulted in better efficacy. Further studies are warranted to evaluate the addition of bevacizumab to monotherapy in elderly patients with advanced non-squamous NSCLC.
Conclusions
Addition of bevacizumab to pemetrexed in previously-untreated elderly patients with non-squamous NSCLC is feasible and results in good QoL and improved efficacy, in terms of PFS, OS, and ORR. Further studies are warranted for establishing the significance of including bevacizumab in the treatment of elderly patients with non-squamous NSCLC. These study data have allowed us to proceed to a phase III study to compare pemetrexed and bevacizumab with standard care.
Acknowledgments
We greatly appreciate for all the participating patients, their families and caregivers, investigators, site staffs, independent data and monitoring committee members and TORG staffs. We also special thanks Elsevier Ltd for Language editing.
Funding: This trial was conducting in Thoracic Oncology Research Group (TORG) (Yokohama, Japan). Chugai Pharmaceutical Co. (Tokyo, Japan) provided the financial support and decided to submit the article for publication.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.29). T Kozuki reports grants and personal fees from Chugai Pharmaceutical Co., AstraZeneca, Taiho, Bristol-Myers Squibb, and Eli Lilly Japan, and personal fees from Ono, MSD, Pfizer Japan, Kyowa Hakko Kirin, Nippon Boehringer Ingelheim, Nippon Kayaku and Novartis, and grants from Merck Biopharma, outside the submitted work. NN reports personal fees from MSD, AstraZeneca, Nikkei Business Publications, Eli Lilly Japan, Pfizer Japan, Bristol-Myers Squibb, Matsuyama Association of Obstetricians and Gynecologists, Reno. Medical, Ehime Medical Association, Ehime University Graduate School of Medicine, Quintiles Transnational Japan and grants and personal fees from Kyowa Hakko Kirin, Taiho, Chugai Pharmaceutical Co, Ono, Nippon Boehringer Ingelheim, outside the submitted work. OH reports personal fees and research funding from Novartis Pharma, Boehringer Ingelheim, AstraZeneca, and research funding from Kyorin Pharmaceutical Co., Bayer Health Care, Daiichi Sankyo, GlaxoSmithKline, and Ono, outside the submitted work. NS reports personal fees from Chugai Pharmaceutical Co., Eli Lilly Japan, and Sanofi, during the conduct of the study; personal fees from AstraZeneca, Boehringer Ingelheim, Taiho, Daiichi Sankyo, Ono, Bristol-Myers Squibb, MSD, and Nihon Medi-Physics, outside the submitted work. TH reports personal fees from Boehringer Ingelheim, Hisamitsu Pharmaceutical Co., and GlaxoSmithKline, outside the submitted work. NF reports personal fees from Chugai Pharmaceutical Co. during the conduct of the study; personal fees from Hisamitsu Pharmaceutical Co., Boehringer Ingelheim, Kyorin Pharmaceutical Co., Ono, and Bristol-Myers Squibb outside the submitted work. AB reports personal fees from Chugai Pharmaceutical Co., and Eli Lilly Japan, during the conduct of the study; personal fees from AstraZeneca, Ono, Bristol-Myers Squibb, Pfizer Japan, Boehringer Ingelheim, Chugai Pharmaceutical Co., Novartis Pharma, and Daiichi Sankyo and research funding from AstraZeneca, Ono, Pfizer, and Abbvie outside the submitted work. KT reports grants from Chugai Pharmaceutical Co., grants from Eli Lilly Japan, outside the submitted work. MS reports grants and personal fees from Chugai Pharmaceutical Co., Eli Lilly Japan, during the conduct of the study; grants and personal fees from Pfizer Japan, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Ono, Novartis, and MSD, personal fees from Taiho, and grants from Ignyta, Abbvie, EPS international, and Takeda, outside the submitted work. T Kato reports grants and personal fees from Chugai Pharmaceutical Co., during the conduct of the study; grants and personal fees from Abbvie, AstraZeneca Bristol-Myers Squibb, Chugai Pharmaceutical Co., Eli Lilly, Kyowa Kirin, Merck Biopharma, MSD, Novartis Ono, Pfizer and Taiho, personal fees from Boehringer Ingelheim, Nitto Denko, Sumitomo Dainippon, Takeda, and F. Hoffmann-La Roche, and grants from Astellas, Kyorin Pharmaceutical Co., and Regeneron, outside the submitted work. TS reports grants and personal fee from MSD and Boehringer Ingelheim, personal fees from Nichi-Iko Pharmaceutical Co., AstraZeneca, Chugai Pharmaceutical Co., Daiichi Sankyo, Ono, Bristol-Myers Squibb, Eli Lilly, Novartis, and Taiho outside the submitted work. HO received research funds from Takeda, MSD, Ono, AstraZeneca, Merck Biopharma, Chugai Pharmaceutical Co., Taiho, Bristol-Myers Squibb, Eli Lilly and Daiichi Sankyo outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspect s of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted according to the principles of Declaration of Helsinki and Good Clinical Practice and approved by the Institutional Review Board of all participating hospitals. All patients enrolled completed the informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Research. FfPoC. Cancer statistics in Japan-2017 2018. Available online: https://ganjoho.jp/reg_stat/statistics/stat/summary.html
- Hanna N, Johnson D, Temin S, et al. Systemic Therapy for Stage IV Non–Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484-515. [Crossref] [PubMed]
- Besse B, Adjei A, Baas P, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol 2014;25:1475-84. [Crossref] [PubMed]
- Kudoh S, Takeda K, Nakagawa K, et al. Phase III Study of Docetaxel Compared With Vinorelbine in Elderly Patients With Advanced Non–Small-Cell Lung Cancer: Results of the West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin Oncol 2006;24:3657-63. [Crossref] [PubMed]
- Abe T, Takeda K, Ohe Y, et al. Randomized Phase III Trial Comparing Weekly Docetaxel Plus Cisplatin Versus Docetaxel Monotherapy Every 3 Weeks in Elderly Patients With Advanced Non–Small-Cell Lung Cancer: The Intergroup Trial JCOG0803/WJOG4307L. J Clin Oncol 2015;33:575-81. [Crossref] [PubMed]
- Langer C, Ravelo A, Hazard SJ, et al. Comparison of survival and hospitalization rates between Medicare patients with advanced NSCLC treated with bevacizumab-carboplatin-paclitaxel and carboplatin-paclitaxel: A retrospective cohort study. Lung Cancer 2014;86:350-7. [Crossref] [PubMed]
- Zhu J, Sharma DB, Gray SW, et al. Carboplatin and paclitaxel with vs without bevacizumab in older patients with advanced non–small cell lung cancer. JAMA 2012;307:1593-601. [Crossref] [PubMed]
- Langer CJ, Socinski MA, Patel JD, et al. Isolating the Role of Bevacizumab in Elderly Patients With Previously Untreated Nonsquamous Non-Small Cell Lung Cancer: Secondary Analyses of the ECOG 4599 and PointBreak Trials. Am J Clin Oncol 2016;39:441-7. [Crossref] [PubMed]
- Crinò L, Dansin E, Garrido P, et al. Safety and efficacy of first-line bevacizumab-based therapy in advanced non-squamous non-small-cell lung cancer (SAiL, MO19390): a phase 4 study. Lancet Oncol 2010;11:733-40. [Crossref] [PubMed]
- Takagi Y, Hosomi Y, Oshita F, et al. Feasibility study of docetaxel plus bevacizumab as first line therapy for elderly patients with advanced non-small-cell lung cancer: Thoracic Oncology Research Group (TORG) 1014. BMC Cancer 2015;15:740-6. [Crossref] [PubMed]
- Kozuki T, Nogami N, Kitajima H, et al. Feasibility study of first-line chemotherapy using Pemetrexed and Bevacizumab for advanced or recurrent nonsquamous non-small cell lung cancer in elderly patients: TORG1015. BMC Cancer 2016;16:306-12. [Crossref] [PubMed]
- Effects of vinorelbine on quality of life and survival of elderly patients with advanced non-small-cell lung cancer. The Elderly Lung Cancer Vinorelbine Italian Study Group. J Natl Cancer Inst 1999;91:66-72. [Crossref] [PubMed]
- Gridelli C, Perrone F, Gallo C, et al. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst 2003;95:362-72. [Crossref] [PubMed]
- Hattori Y, Iwasaku M, Satouchi M, et al. A Phase II Study of Pemetrexed in Chemotherapy-naïve Elderly Patients Aged ≥75 years with Advanced Non-squamous Non-small-cell Lung Cancer (HANSHIN Oncology Group 003). Jpn J Clin Oncol 2013;43:1184-9. [Crossref] [PubMed]
- Kim YH, Hirabayashi M, Kosaka S, et al. Phase II study of pemetrexed as first-line treatment in elderly (≥75) non-squamous non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0901. Cancer Chemother Pharmacol 2013;71:1445-51. [Crossref] [PubMed]
- Schuette W, Schneider CP, Engel-Riedel W, et al. 65Plus: open-label study of bevacizumab in combination with pemetrexed or pemetrexed/carboplatin as first-line treatment of patients with advanced or recurrent nonsquamous non-small-cell lung cancer. Lung Cancer (Auckl) 2017;8:217-29. [Crossref] [PubMed]
- Takeoka H, Yamada K, Naito Y, et al. Phase II Trial of Carboplatin and Pemetrexed Plus Bevacizumab with Maintenance Bevacizumab as a First-line Treatment for Advanced Non-squamous Non-small Cell Lung Cancer in Elderly Patients. Anticancer Res 2018;38:3779-84. [Crossref] [PubMed]
- Herbst RS, O'Neill VJ, Fehrenbacher L, et al. Phase II Study of Efficacy and Safety of Bevacizumab in Combination With Chemotherapy or Erlotinib Compared With Chemotherapy Alone for Treatment of Recurrent or Refractory Non–Small-Cell Lung Cancer. J Clin Oncol 2007;25:4743-50. [Crossref] [PubMed]
- Spigel DR, Hainsworth JD, Joseph MJ, et al. Randomized phase 2 trial of pemetrexed, pemetrexed/bevacizumab, and pemetrexed/carboplatin/bevacizumab in patients with stage IIIB/IV non–small cell lung cancer and an Eastern Cooperative Oncology Group performance status of 2. Cancer 2018;124:1982-91. [Crossref] [PubMed]