
Determining the invasiveness of pure ground-glass nodules using dual-energy spectral computed tomography
Introduction
With the application of high-resolution computed tomography (CT), more and more ground-glass opacity nodules are being detected. Ground-glass nodule (GGN) on CT is defined as hazy increased density of the lungs, but with presentation of bronchial and vascular margins (1). GGN is classified into pure ground-glass nodule (pGGN), which has no solid components, and part-solid nodules (PSNs), with solid components (2). In some articles, the ratio of solid components is studied, and the more the solid components are included, the more malignancy it may be (3,4).
Though many diseases either benign or malignancy can manifest as GGN, it is reported that lung adenocarcinoma often appears as localized GGNs on CT, and lung adenocarcinoma is becoming the main pathology type of lung cancer. According to the classification on lung adenocarcinoma proposed in 2011 (5) and adopted by WHO in 2015 (6), adenocarcinoma is classified into atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA) and invasive adenocarcinoma (IA). On one hand, the AIS and MIA had similar prognoses, with 100% disease-specific survival (5). While for IA, the 5-year overall survival is about 49–84% (7). On the other hand, even though the resection for the entire group of lymph node or lobectomy is still the standard surgical treatment of IA, patients with AIS or MIA can accept wedge or segment resection to maintain lung function and reduce incidence and mortality. For pGGN, approximately 30–40% of all resected pGGNs were IAs in some studies (8-10). Therefore, it is important to be able to differentiate the invasiveness of pGGNs.
In recent years the dual-energy spectral CT, which extends the capabilities of conventional CT, has emerged as a promising non-invasive imaging method in clinical work. It provides material decomposition images and virtual monochromatic images for quantification, the parameters including iodine concentration (IC) and water content (WC) from material decomposition images, CT attenuation value (CT number) measurements from the 101 sets of virtual monochromatic images (40–140 keV photon energy) (11). Many researchers have already investigated the value of GGN morphology (12-16) and several quantitative CT features for predicting the invasiveness of lung pure ground-glass nodules on conventional CT (17). In addition, researchers have already used quantitative CT parameters (i.e. volume doubling time and mass doubling time) for predicting the behavior of persistent GGNs and the controversial role of doubling time (DT) in predicting the invasiveness of adenocarcinomas presenting as GGNs (18-20). However, there are few research about using dual-energy spectral CT to analyze GGN, especially about pGGN. So, the purpose of our study was to investigate the clinical application of using the quantitative parameters generated in the unenhanced phase and venous phase in dual-energy spectral CT for differentiating the invasiveness of pure ground-glass nodules (pGGNs).
Methods
Subjects
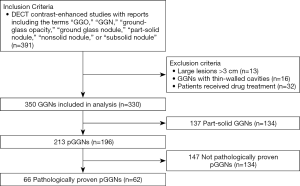
We retrospectively analyzed 62 patients with 66 pure ground-glass nodules (58 patients with single lesion, 4 patients with 2 lesions) who underwent both the unenhanced and the contrast-enhanced dual-energy spectral CT from April 2016 to May 2019 in our hospital (Figure 1 shows the flowchart of study population). These patients were identified by pathology as having pGGN. Of the 62 patients, 43 were females and 19 were males, with age ranging from 29 to 75 years and mean age of 55.4 years. The inclusion criteria were: (I) nodules were pathologically confirmed and without solid components; (II) nodules with diameters of 3 cm or less in their largest dimension (lesion size was measured on thin-section images).
CT examination
The unenhanced and contrast-enhanced dual-energy spectral CT scans of the lungs were performed on a 64-slice CT scanner (Discovery CT750HD, GE Healthcare, Waukesha USA) using the following scan protocol: (I) spectral imaging mode with single-source, fast tube voltage switching between 80 and 140 kVp on adjacent views; (II) 0.6 seconds tube rotation time; (III) 275 mA tube current; (IV) 1.375:1 helical pitch; (V) 500 mm scan field of view; (VI) 1.25 mm slice thickness and image interval. For the contrast-enhanced CT scan, all patients were injected with non-ionic iodinated contrast material with 370 mg/mL concentration (Iopamidol, Shanghai Bracco Sine Pharmaceutical, Shanghai, China) at a dose of 1.35 mL/kg body weight and a contrast injection rate of 3.0 mL/s by using a power injector (OptiVantage, Tyco Healthcare, Cincinnati). The venous phase (VP) scan started at 65 seconds after the beginning of contrast injection.
Imaging analysis
Two types of images were reconstructed from the single dual-energy spectral CT scan: iodine and water-based material decomposition images and 101 sets of virtual monochromatic images with photon energies from 40–140 keV at 1 keV interval. These images were transferred to an advanced workstation (ADW4.6; GE Healthcare) with Gemstone Spectral Imaging (GSI) Viewer software (GE Healthcare) for analysis.
Two radiologists unaware of patients’ information and pathologic results interpreted the images and measured the quantitative parameters on the monochromatic and material decomposition images in the unenhanced phase and venous phase on ADW4.6 independent of each other. The principles for the measurement of pGGN were as following: (I) Selecting three slices containing pGGN; (II) selecting a region-of-interest (ROI) covering more than 70% of the lesion area in the maximum section for the lesion and avoid major vessels and bubbles. The size, shape, and position of the ROIs in different image types and phases were kept consistent using the copy and paste function as far as possible. Measurements were performed three times in the three consecutive axial sections to obtain average values for the individual reviewer. And finally, the average values for the two reviewers were calculated and used for analysis and comparison among different lesion types. The measurements included the CT attenuation values (CT numbers) of pGGN using the 40–140 keV virtual monochromatic images and their iodine concentration (IC) and water content (WC) from the iodine-based and water-based material decomposition images, respectively in both the unenhanced and venous phases. To minimize the influence of the individual circulation status and scanning times, the IC values of lesions were normalized to that of the aorta in the same section to calculate the normalized iodine concentration (NIC): NIC = IClesion/ICaorta. The slope(k) of the CT number as function of photon energy curve was calculated using the 101 sets of monochromatic images: slope(k) = |CT number (40 keV) − CT number (100 keV)|/60.
Statistical analysis
All data were analyzed using a commercial statistical software package (version 22.0, SPSS IBM; MedCalc, version 12.0, MedCalc Software, Mariakerke, Belgium; GraphPad Prism version 6, GraphPad Software, La Jolla, California, USA), A P value of <0.05 was considered statistically significant.
In all, the maximum diameter of nodules, IC, NIC, the slope(k), WC and CT numbers of monochromatic images at 40–140 keV in two phases were obtained. The results were expressed as mean ± standard deviation. In addition, because AIS and MIA had similar prognoses, these two subtypes of pGGNs were combined into AIS-MIA group and compared with IA group. Normality of variance was assessed by the one-sample Kolmogorov-Smirnov (K-S) test. For normally distributed continuous variables, independent-samples t-test was used. Non-normally distributed continuous variables were analyzed by the non-parametric K-S test. The significant factors in the univariate analysis were identified as candidate covariates in a logistic regression model with forward stepwise selection, and odds ratios (ORs) were calculated. Furthermore, receiver operating characteristic (ROC) analysis was performed to evaluate the performance of spectral CT parameters in differentiating the AIS-MIA group from IA group.
Results
Patients
Sixty-two patients underwent dual-energy spectral CT examination were included in the study. There were 66 pGGNs (58 patients with single lesion, 4 patients with 2 lesions) identified by pathology after CT examination. Among the 66 pGGNs, 19 were AIS, 22 were MIA and 25 were IA. The clinical characteristics of the 62 patients are summarized in Table 1.

Full table
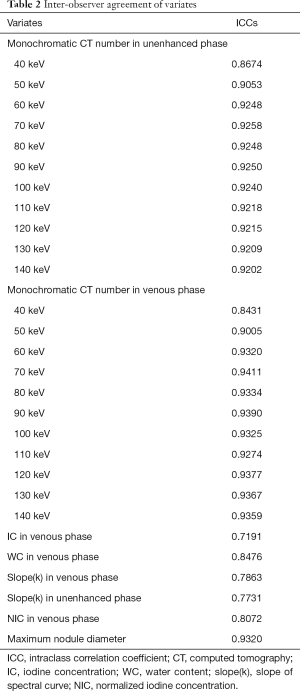
Inter-observer agreement
Interobserver agreement for measurements is shown in Table 2.
Full table
Relationships between pathology and Dual-energy spectral CT findings
The CT numbers at 40–140 keV in both phases had statistically significant difference between preinvasive lesion group and IA group (Tables 3,4).

Full table

Full table
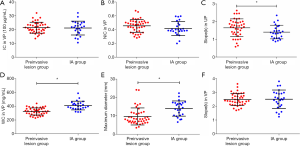
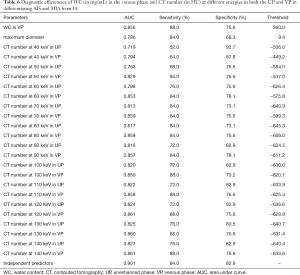
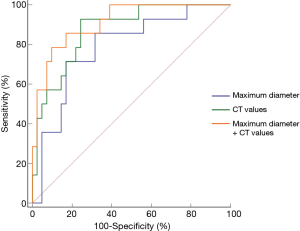
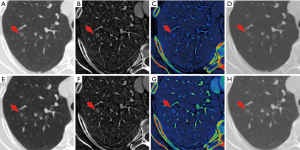
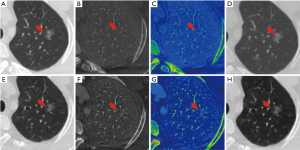
The comparison of the material density measurements of pGGNs with different invasiveness is shown in Table 5 and Figure 2. There were significant differences in the water content in venous phase, and the maximum diameter of pGGNs between preinvasive lesion group and IA group. However, there was no difference in both the iodine concentration and normalized iodine concentration between them. For multivariate analysis, we used the parameters that were statistically significant in the univariate analysis. The results revealed that the maximum nodule diameter (odd ratio OR =1.21, 95% CI: 1.050–1.400, P<0.01) and the CT number in the 130keV images in venous phase (OR =1.03, 95% CI: 1.014–1.047, P<0.001) were independent predictors for the IA group. The area under the curve (AUC), sensitivity and specificity of the predictive model were 0.901, 84.00%, 82.93%, and showing higher AUC value than the maximum nodule diameter (AUC=0.786), or the CT number of the 130 keV images in venous phase (AUC=0.860) alone (Table 6 and Figure 3). The two clinical cases are shown in Figures 4 and 5.

Full table
Full table
Discussion
The quantitative enhancement degree of lesion in conventional CT can reflect the blood supply and distribution in the intravascular and extracellular spaces and assess the benign or malignancy of lesions (21,22). With the development of technology, dual-energy spectral CT provides not only material concentrations using material decomposition images to better reflect the contrast-enhancement information, but also CT attenuation values from a set of virtual monochromatic images to be less influenced by beam hardening effects as in the conventional CT. The material decomposition images can be used to estimate quantitatively the water and iodine content (23) in lesions. In addition, monochromatic image sets provide information about the attenuation changes of different materials as a function of X-ray photon energy. However, a few researchers have analyzed the GGN using dual-energy spectral CT. Son et al. (24) found that combining texture analyze with dual-energy spectral CT, the iodine-enhanced imaging metrics versus virtual non-contrast (VNC) imaging metrics have added value in distinguishing IA from AIS or MIA. But it was difficult and tedious in clinical application. Recently, Liu et al. (25) confirmed that tumorous GGN has blood supply. But they didn’t analyze the invasiveness of GGNs. Other researchers (26) studied the CT attenuation of pGGN in a series of enhanced monochromatic energies and found that monochromatic CT number of higher energy (especially 140 keV) would be better for diagnosing IA than lower energies. Whereas they only analyzed the delayed phase images. Yang et al. (27) proposed modified NIC to differentiating IAs from preinvasive lesions using a dual-source, dual-energy approach, and the study design was rather complicated. Unlike those previous studies, we further performed quantitative analysis of iodine concentration, normalized iodine concentration, water content and monochromatic CT numbers in both the unenhanced phase and venous phase using a single-source dual-energy approach. Similar to the previous studies (28), we found that the most common sub-types of pGGN in our study were AIS and MIA.
Histologically, GGN is the partial filling of the terminal air spaces, interstitial thickening by fluid, cells, fibrosis and increased capillary blood volume, or a combination of these factors (29,30). Interestingly, we found that IC and NIC in the venous phase had no significance in indicting the degrees of invasion. The iodine concentration is often used to reflect the blood flow in contrast-enhanced CT scans, since iodine is the most commonly used contrast agent (31). The no difference finding in IC may indicate that the blood supply to pGGN of different types was not high enough or different enough to be differentiated.
On the other hand, the intrinsic structures or densities of pGGNs with different invasiveness may be different. In our study, we used the iodine-water base material pairs to represent the attenuation of nodules in the enhancement phase. The combination of these two materials reflected the detected density. Since iodine better represents the contrast medium that is in the nodule, the water content reflects the physical density of the GGN (32). Similarly to the previous study (25), we found that the water content in both the unenhanced and contrast-enhanced venous phases had significantly differences between the preinvasive lesion group and IA group, and the IA group had the highest water content. Travis et al. (5) pointed out that tumor cells in pGGNs are usually distributed along pre-existing lung alveolar structures in a lepidic growth pattern, which present looseness. With the invasion of stroma, stromal proliferation, or framework collapse, the lesions become more compact. Since IA has more tumor cells proliferation and invasion, it is thus reasonable that IA is more compact and has higher density, thus higher water content in IA than in the pre-invasion nodules.
In our study, the maximum nodule diameter was shown to be an independent factor in discriminating IA with the cut-off value of 9.36 mm. In general, our findings are similar to the results of Lee et al. (28) and Jin et al. (9) that pGGNs with diameters of 10 or 10.5 mm are more likely to be IA, but a size measurement alone may not be reliable enough to indicate histopathologic subtypes.
One of the advantages of dual-energy spectral CT is that it produces a set of virtual monochromatic images from 40 to 140 keV photon energies and allows image interpretation at various energy levels from a single CT examination. Our results indicated that there were statistically significant differences in the CT number in the entire energy spectrum between the preinvasive lesion group and IA group. We also found that the CT numbers of the higher energy monochromatic images had high diagnostic power in indicating IA. This finding is similar to that of Zhang et al. (26), whose study showed that the CT numbers of the 120–140 keV monochromatic images in the contrast-enhanced delay phase performed better than the CT numbers of the conventional nonenhanced polychromatic images to indicate IA. In dual-energy spectral CT, organs or lesions containing iodine appear denser in the low-keV images, but the image noise can also be much higher despite contrasting with the surrounding tissue is evident (33). On the other hand, the monochromatic images with high energy have better noise performance (34,35), and it is thus advantageous to be able to fully utilize high energy monochromatic images. We found that slope(k) in the unenhanced phase had significant difference in distinguishing IA group from preinvasive lesion group. In addition, we also showed that the monochromatic CT number had significant difference in distinguishing IA group from preinvasive lesion group in both the unenhanced phase and venous phase with dual energy CT, in the multivariate analysis, we found only CT numbers in the 130 keV images in venous phase was the predicting factor for IA.
There are some limitations in our study. Firstly, our results were limited by the relatively small sample size. More patients, especially patients with IA are needed in future studies to improve the accuracy. Secondly, we only analyzed the maximum nodule diameter, CT number and material density parameters of pGGN, more morphology information will need to be added in the future. Thirdly, this was a retrospective and single institution study, and there existed patient selection bias.
Conclusions
Overall, the quantitative parameters in dual-energy spectral CT in the unenhanced phase and venous phase, together with the maximum diameter of lesions provide useful information in predicting IA, which manifested as pure ground-glass nodules.
Acknowledgments
Funding: This research was supported by the National Natural Science Foundation of China (Grant No.81571670).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.33). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This research was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research was approved by the Institutional Ethics Board of Renji Hospital, School of Medicine, Shanghai Jiaotong University (No. 2017-082) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Henschke CI, Yankelevitz DF, Mirtcheva R, et al. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am J Roentgenol 2002;178:1053-7. [Crossref] [PubMed]
- Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697-722. [Crossref] [PubMed]
- Moon Y, Lee K, Park J. The prognosis of invasive adenocarcinoma presenting as ground-glass opacity on chest computed tomography after sublobar resection. J Thorac Dis 2017;9:3782-92. [Crossref] [PubMed]
- Wu H, Liu C, Xu M, et al. A Retrospective Study of Mean Computed Tomography Value to Predict the Tumor Invasiveness in AAH and Clinical Stage Ia Lung Cancer. Zhongguo Fei Ai Za Zhi 2018;21:190-6. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Van Schil PE, Asamura H, Rusch VW, et al. Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur Respir J 2012;39:478-86. [Crossref] [PubMed]
- Lim HJ, Ahn S, Lee KS, et al. Persistent pure ground-glass opacity lung nodules ≥ 10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 2013;144:1291-9. [Crossref] [PubMed]
- Jin X, Zhao SH, Gao J, et al. CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground-glass opacity. Eur Radiol 2015;25:2532-40. [Crossref] [PubMed]
- Li Q, Fan L, Cao ET, et al. Quantitative CT analysis of pulmonary pure ground-glass nodule predicts histological invasiveness. Eur J Radiol 2017;89:67-71. [Crossref] [PubMed]
- Aoki M, Takai Y, Narita Y, et al. Correlation between tumor size and blood volume in lung tumors: a prospective study on dual-energy gemstone spectral CT imaging. J Radiat Res 2014;55:917-23. [Crossref] [PubMed]
- Ding H, Shi J, Zhou X, et al. Value of CT Characteristics in Predicting Invasiveness of Adenocarcinoma Presented as Pulmonary Ground-Glass Nodules. Thorac Cardiovasc Surg 2017;65:136-41. [PubMed]
- Si MJ, Tao X, Du G, et al. Thin-section computed tomography-histopathologic comparisons of pulmonary focal interstitial fibrosis, atypical adenomatous hyperplasia, adenocarcinoma in situ, and minimally invasive adenocarcinoma with pure ground-glass opacity. Eur J Radiol 2016;85:1708-15. [Crossref] [PubMed]
- Wu F, Tian S, Jin X, et al. CT and histopathologic characteristics of lung adenocarcinoma with pure ground-glass nodules 10 mm or less in diameter. Eur Radiol 2017;27:4037-43. [Crossref] [PubMed]
- Zhang Y, Qiang J, Ye J, et al. High resolution CT in differentiating minimally invasive component in early lung adenocarcinoma. Lung Cancer 2014;84:236-41. [Crossref] [PubMed]
- Yue X, Liu S, Liu S, et al. HRCT morphological characteristics distinguishing minimally invasive pulmonary adenocarcinoma from invasive pulmonary adenocarcinoma appearing as subsolid nodules with a diameter of</=3 cm. Clin Radiol 2018;73:411.e7-411.e15. [Crossref] [PubMed]
- Han L, Zhang P, Wang Y, et al. CT quantitative parameters to predict the invasiveness of lung pure ground-glass nodules (pGGNs). Clin Radiol 2018;73:504.e1-504.e7. [Crossref] [PubMed]
- Song YS, Park CM, Park SJ, et al. Volume and mass doubling times of persistent pulmonary subsolid nodules detected in patients without known malignancy. Radiology 2014;273:276-84. [Crossref] [PubMed]
- Borghesi A, Farina D, Michelini S, et al. Pulmonary adenocarcinomas presenting as ground-glass opacities on multidetector CT: three-dimensional computer-assisted analysis of growth pattern and doubling time. Diagn Interv Radiol 2016;22:525-33. [Crossref] [PubMed]
- Kim H, Goo JM, Park CM. Evaluation of T categories for pure ground-glass nodules with semi-automatic volumetry: is mass a better predictor of invasive part size than other volumetric parameters? Eur Radiol 2018;28:4288-95. [Crossref] [PubMed]
- Zhang Y, Cheng J, Hua X, et al. Can Spectral CT Imaging Improve the Differentiation between Malignant and Benign Solitary Pulmonary Nodules? PLoS One 2016;11:e0147537. [Crossref] [PubMed]
- Chae EJ, Song JW, Seo JB, et al. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: initial experience. Radiology 2008;249:671-81. [Crossref] [PubMed]
- Fischer MA, Gnannt R, Raptis D, et al. Quantification of liver fat in the presence of iron and iodine: an ex-vivo dual-energy CT study. Invest Radiol 2011;46:351-8. [Crossref] [PubMed]
- Son JY, Lee HY, Kim JH, et al. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol 2016;26:43-54. [Crossref] [PubMed]
- Liu G, Li M, Li G, et al. Assessing the Blood Supply Status of the Focal Ground-Glass Opacity in Lungs Using Spectral Computed Tomography. Korean J Radiol 2018;19:130-8. [Crossref] [PubMed]
- Zhang Y, Tang J, Xu J, et al. Analysis of pulmonary pure ground-glass nodule in enhanced dual energy CT imaging for predicting invasive adenocarcinoma: comparing with conventional thin-section CT imaging. J Thorac Dis 2017;9:4967-78. [Crossref] [PubMed]
- Yang Y, Li K, Sun D, et al. Invasive Pulmonary Adenocarcinomas Versus Preinvasive Lesions Appearing as Pure Ground-Glass Nodules: Differentiation Using Enhanced Dual-Source Dual-Energy CT. AJR Am J Roentgenol 2019;213:W114-22. [Crossref] [PubMed]
- Lee SM, Park CM, Goo JM, et al. Invasive Pulmonary Adenocarcinomas versus Preinvasive Lesions Appearing as Ground-Glass Nodules: Differentiation by Using CT Features. Radiology 2013;268:265-73. [Crossref] [PubMed]
- Lee HY, Choi Y-L, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol 2014;202:W224-33. [Crossref] [PubMed]
- Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics 2007;27:391-408. [Crossref] [PubMed]
- McCollough CH, Leng S, Yu L, et al. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015;276:637-53. [Crossref] [PubMed]
- Thieme SF, Johnson TR, Lee C, et al. Dual-energy CT for the assessment of contrast material distribution in the pulmonary parenchyma. AJR Am J Roentgenol 2009;193:144-9. [Crossref] [PubMed]
- Park J, Choi YH, Cheon JE, et al. Advanced virtual monochromatic reconstruction of dual-energy unenhanced brain computed tomography in children: comparison of image quality against standard mono-energetic images and conventional polychromatic computed tomography. Pediatr Radiol 2017;47:1648-58. [Crossref] [PubMed]
- Wang Y, Qian B, Li B, et al. Metal artifacts reduction using monochromatic images from spectral CT: evaluation of pedicle screws in patients with scoliosis. Eur J Radiol 2013;82:e360-6. [Crossref] [PubMed]
- Horat L, Hamie MQ, Huber FA, et al. Optimization of Monoenergetic Extrapolations in Dual-Energy CT for Metal Artifact Reduction in Different Body Regions and Orthopedic Implants. Acad Radiol 2019;26:e67-e74. [Crossref] [PubMed]


