
Clinical significance of histologic subtyping of malignant pleural mesothelioma
Introduction
Malignant mesothelioma (MM) is a rare and, in majority of cases, highly aggressive tumor. It arises from the mesothelial cells lining serous cavities (pleura, pericardium, peritoneum, and tunica vaginalis). In Western countries, incidence of MM is still slowly increasing, and should be reaching its peak in this decade (1-5). Without treatment, median survival is 7 to 9 months, and even with treatment it is no more than 22 months (6). Despite advances in multimodality treatment options (7-9), 5-year-survival is only 5% (10,11).
The histologic subtype of MM and TNM stage remain the major prognostic factors (12). It is well known that epithelioid subtype is associated with better prognosis when compared to sarcomatoid and biphasic subtypes (13). The patients with epithelioid mesothelioma may benefit from surgical treatment, while the patients with sarcomatoid mesothelioma do not show the same benefit and therefore are not subjected to surgery. Furthermore, the epithelioid subtype shows a large spectrum of morphological heterogeneity that is nicely described and illustrated in the 2015 WHO classification (13). Recently published studies demonstrated that morphological subtype of epithelioid mesothelioma does have an impact on outcome (14-16). On the other hand, in biphasic mesothelioma the proportion of sarcomatoid component seems to be the major driver of prognosis (17,18). These are just a few examples of how morphology may have an impact on prognosis and treatment decision. It remains to be determined how many details in regards to morphology of MMs have to be mentioned in the diagnostic pathology reports. In addition to architecture of mesothelial subtypes, recent studies indicate that cytologic features, mitoses and necrosis may be translated into grading score that also has prognostic significance (19,20). Currently there is no recommendation on grading of epithelioid mesotheliomas, but that may change in the near future.
In contrast to other tumor types such as lung cancer, MMs show rather a limited number of genomic alterations and usually lack of druggable targets. Recent developments in immunotherapy for MMs suggest that histology may be predictor of response. Therefore, it remains to be seen if precise histological subtyping and grading of mesothelioma would be of any predictive value for novel non-surgical therapeutic approaches.
In this review, major histologic subtypes and cytological features of MM are presented and their relation to prognosis and possible predictive value is discussed.
Well-differentiated papillary mesothelioma (WDPM)
WDPM is a localized or multifocal tumor. It is exceptionally rare in pleura, and occurs more often in peritoneum and tunica vaginalis testis (21,22). Histologically, it is defined by papillary growth pattern, where one layer of cytologically bland, epithelioid cells covers papillae. Fibrovascular cores of papillae often show myxoid changes. Atypia and mitoses are absent. WDPM most commonly grows superficially. In rare cases, focal and superficial invasion can be present as a stalk invasion with bland-looking cells or presenting as solid aggregates with cytological higher grade. In such case, term “WDPM with invasive foci” is applied and closer follow-up due to recurrence is needed (23).
Few molecular studies published up to date demonstrated NF2 heterozygous deletion, E2F1 point mutation and one case of germline BAP1 mutation (24-26), characterizing WDPM as a neoplastic process. Recently, Stevers et al. showed that peritoneal WDPM harbors mutually exclusive somatic missense mutations in TRAF7 or CDC42 genes, while no alterations in BAP1, NF2, CDKN2A, DDX3X, SETD2, and ALK genes have been found (27). Since the prognosis is very good, with occasional local recurrences, differentiation from diffuse epithelioid MM with predominant papillary pattern is crucial. The main characteristic features favoring WDPM are monomorphic histological presentation with only one (papillary) pattern, single layer of cells, low mitotic count, and absence of atypia and invasion. WDPM has intact BAP1 nuclear expression, and no homozygous deletion of CDKN2A/p16, therefore these features can be helpful in the differential diagnosis with diffuse MM. Reactive mesothelial proliferation is another diagnostic pitfall, however it is characterized by thinner papillae and hyalinized fibrovascular cores with prominent blood vessels. In this differential diagnostic situation morphology trumpets over ancillary methods. Radiologic correlation is always needed, and the diagnosis on a small biopsy is often challenging.
Localized malignant mesothelioma (LMM)
LMM is defined as a solitary, nodular lesion, without diffuse involvement of the serosal surface, both macroscopically and histologically. Thorough radiological investigations and thoracoscopic inspection is necessary to be able to confirm the diagnosis of LMM. It presents in 3 histologic types: epithelioid, sarcomatoid and biphasic (28,29). Genetically they are heterogeneous, and some have BAP-1 mutations like DMM, but others have mutations, like TRAF7, which are more specific for LMM (30).
Although LMM is very rare, it has to be recognized, as the prognosis of this unique type is much better than for diffuse MM, and it is potentially curable by complete surgical excision (28,31).
Diffuse malignant mesothelioma (DMM)
DMM is according to the 2015 WHO classification divided in epithelioid, sarcomatoid and biphasic subtypes (Figure 1) (13). Importance of its recognition lies in the fact that the overall prognosis of the patients with DMM is very poor, even when compared to LMM, and especially to WDPM. Major diagnostic challenges are in the recognition of reactive lesions, recently described in-situ mesotheliomas and in differentiation from the most important differential diagnostic possibilities, such as carcinomas and spindle cell lesions/sarcomas. Diagnosis relies on morphological criteria, as well as on immunohistochemistry, and sometimes even molecular analyses is needed. However, it is out of the scope of this review to go into this problematic diagnostic challenges. In the next paragraphs, we will shortly explain the importance of recognizing specific histologic types and additional cytological and stromal features.
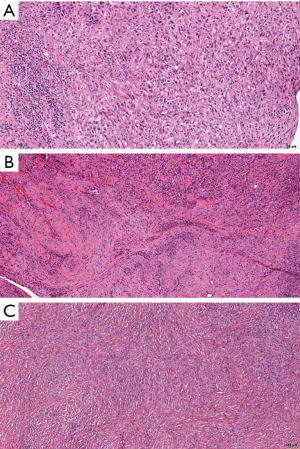
Epithelioid malignant mesothelioma (EMM)
EMM, found in up to 80% of patients with MM, is characterized by diffuse and invasive growth of epithelioid cells from pleural surface. It has been known for its heterogeneous morphology. According to the 2015 WHO Classification, patterns that occur more often are solid, tubulopapillary, and trabecular, followed by micropapillary, adenomatoid, clear cell, transitional, deciduoid, and small cell (13). Furthermore, EMM usually has more than two growth patterns, and this feature can be used as a diagnostic criterion versus some other malignant tumors. Recognition of different growth pattern is important since distinct subtypes have prognostic importance. In a study by Kadota et al. (14) trabecular and tubulopapillary pattern proved to be favorable prognostic patterns, in comparison to other patterns in EMM. The same was true for the myxoid and microcystic pattern in another study (15). In contrast, pleomorphic pattern was found to be associated with poor survival, more similar to the patients with sarcomatoid malignant mesothelioma (SMM) (14,16,32). Pleomorphic characteristic can also occur in SMM (33). Recently described transitional pattern is characterized by sheets of round to oval malignant mesothelial cells with abundant cytoplasm, morphologically lying between epithelioid and spindle cells (17,34). It is associated with a survival similar to that of the sarcomatoid and pleomorphic types. Furthermore, molecular characteristics are also similar to SMM (Galateau Salle F et al, 2020, submitted for publication). Some authors regarded lymphohistiocytoid mesothelioma as a separate, uncommon subtype of SMM (35), while others, based on a better prognosis, considered this pattern as a part of the epithelioid subtype (36). Of note, a very small number of lymphohistiocytiod mesothelioma cases has been reported in the literature, sometimes with better, and sometimes with worse survival data (37). The similar problem of (under)representativeness have another three mesothelioma types/patterns, namely deciduoid mesothelioma, signet ring and small cell mesothelioma. All of them are extremely rare, and it is difficult to define them, in a sense of avoiding misdiagnosis and providing adequate diagnostic reproducibility. Deciduoid type was first described in 1985 by Talerman et al. (38) in the peritoneum, and later in the pleura. However, less than 50 cases of pleural deciduoid mesotheliomas have been published up to date, and prognosis is closer to EMM than to sarcomatoid one (39). Small cell variant of MM is rarer, first reported in series of 13 cases by Mayal and Gibbs in 1992 (40), characterized by similar cell morphology to SCLC, but different immunohistochemical profile (negative for carcinoma markers, as well as chromogranin and synapthophysin, positive for mesothelial markers, and occasionally and focally for CD56). The prognosis is poor, with reported mean survival of 8.2 months (41). Signet ring cell variant seems to be even rarer than previous two variants, with so far less than 30 reported cases, majority involving the pleura (42). Median survival was 15 months (43), and the major diagnostic challenge is ruling out the metastasis.
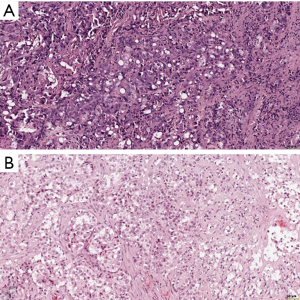
In the recently published EURACAN/IASLC proposal for histologic classification of pleural mesothelioma (44), consensus was made to report the following histologic patterns: tubular, papillary, tubulopapillary, trabecular, solid, micropapillary, adenomatoid, microcystic, pleomorphic and transitional (Figures 2,3). These patters should be reported in percentages in the resected specimens, and mentioned in the report of smaller samples. Other characteristics, such as rhabdoid, deciduoid, small cell, clear cell, signet ring cell and lymphohistiocytoid, are classified as cytological features, and should be reported as such (Figure 4). Myxoid stroma should be reported in EMM when present in more than 50% of tumor, in which less than 50% of tumor cells grow in a solid growth pattern, while it is a favorable prognostic factor.
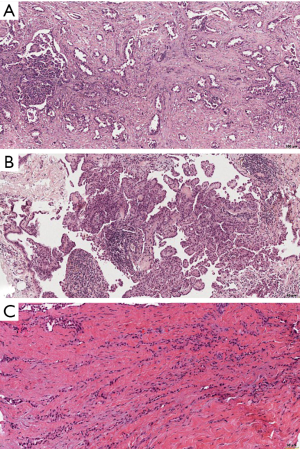
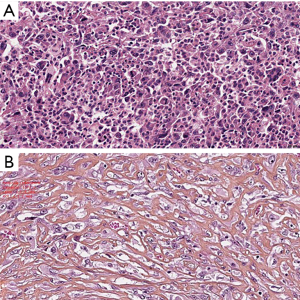
SMM
SMM is defined by diffuse and infiltrative growth of spindle cells, or mesenchymal appearing cells. A special subtype, with at least 50% of tumor mass composed of dense hyalinized stroma that is interspersed between malignant mesothelial cells, is called desmoplastic malignant mesothelioma (13). SMM and desmoplastic MM are very rare, comprising less than 10%, and less than 2%, of all mesothelioma patients, respectively (13). In contrast to other MM, SMM and desmoplastic subtype usually present without pleural effusion, and often have more distant metastases. The latter is especially true for desmoplastic MM, in which distant metastases might occur in up to 60% of patients (45). Both are very challenging for diagnosis, especially in a small biopsy specimen, and major differential diagnosis includes sarcomas (for SMM) and fibrosing pleuritis (for desmoplastic malignant mesothelioma). Pronounced atypia of tumor cells, necrosis and clear invasion are helpful for the diagnosis. The tumor cells can show a wide range of atypia, varying from minimal to severe, with pleomorphic features like atypical giant cells, bizarre nuclei, with presence of atypical mitotic figures. Furthermore, usual mesothelial immunohistochemical markers in these tumors may not be helpful. Tumor cells usually stain for cytokeratins, and GATA 3 shows strong nuclear staining (46). However, the right diagnosis is very important for the evaluation of prognosis and adequate treatment decision. Klebe et al. (47) suggested a separate subtype of mesothelioma with heterologous elements (osteosarcomatous, chondrosarcomatous, rhabdomyosarcomatous and rarely liposarcomatous). They presented 27 mesotheliomas with heterologous elements, 16 were SMM, 10 BMM and 1 diagnosed as EMM (in a small biopsy). Their prognosis proved to be very poor, with median survival of 6 months, and only 1 patient survived longer than 1 year (47). In the literature, there are some reports of very long survival of these patients, one reaching even 69 months (48,49). However, heterologous elements occur extremely rare, in less than 0.5% of all MM, and the consensus proposal of EURACAN/IASLC included these element as stromal features, together with desmoplastic stroma (44).
Prognosis is extremely poor- untreated patients with SMM die of disease within 5–6 months, and majority of patients with desmoplastic malignant mesothelioma have similar or slightly shorter survival time (12,50,51). As mentioned previously, published data clearly demonstrate similar survival of patients with pleomorphic and transitional patterns, however, due to small number of patients and published studies, they are still included both under EMM and SMM (44).
Biphasic malignant mesothelioma (BMM)
BMM is characterized by having at least 10% of each, epithelioid and sarcomatoid component. It comprises 10–15% of all DMM, and prognosis lies between pure epithelioid and sarcomatoid MM (13). Some authors suggest that the amount of sarcomatoid elements is crucial for prognosis. It is known that patients with EMM might profit from surgical procedures, while SMM have very poor prognosis and surgery does not improve survival. Because of these features, it is logical to presume that the amount of epithelioid part in BMM has prognostic role, and therefore influence therapy decisions. However, studies analyzing this are rare, and differ in the proposed cut-off values (17,18). Vigneswaran et al. demonstrated that patients with less than 50% of epithelioid component have very poor survival (6.62 months) in comparison to patients with more than 50% (11.8 months) or patients with pure EMM (20.1 months). Epithelioid component in that study was independent predictor of survival (18). Experts of the French Mesothelioma Panel found better overall survival in patients with more than 20% of epithelioid component. Another study performed by the International Mesothelioma Panel, showed how difficult it is to recognize BMM, reaching only moderate interobserver agreement in diagnosis (weighted kappa value of 0.45) (17). The main problem was identification of a spindle cell component as malignant. BAP1 loss, and CDKN2A/p16 homozygous deletion were in these instances helpful. Furthermore, grading of the spindle cell component, may have prognostic significance. Additionally, the importance of cytokeratin staining in diagnosing BMM was also demonstrated (17). It is known that the accuracy of histologic classification made on small samples is not ideal, and concordance with diagnosis of surgical resection is in range from 72–83% (52,53). Especially underdiagnosed in small biopsies are BMM, while EMM are overdiagnosed. One reason is definitely the sampling issue, and another is strict criteria of 10% of either component. According to the EURACAN/IASLC proposal for histologic classification of pleural mesothelioma consensus paper, definition for BMM should be changed in a way that it can be diagnosed in small samples even without reaching currently set cut-off values of 10% (44).
Biomarker testing in MM
Currently, there is no routinely used testing for prognostic or predictive biomarkers for MM. Emerging data suggest that the histologic type of mesothelioma show different associations with different predictive biomarkers.
PD-L1 is very interesting, since it is used also as a predictive biomarker for immunotherapy, which is now standard therapy for many different solid tumors. In published studies, PD-L1 expression was related to poor prognosis in MM patients (54-58). In a study by Nguyen et al. the difference in median survival of MM patients, depending on PD-L1 expression and regardless of histology, was 9.5 months in favor of negative PD-L1 expression (58). Their study, as well as other studies, showed also that PD-L1 expression is associated with SMM (57,59).
The same was confirmed by the one of the largest study so far, using 214 samples of MAPS phase 3 randomized trial (60). Thirty-five point nine percent [77] samples were PD-L1 positive, out of those 35.1% (27 samples) had 50% or more positive tumor cells. SMM and BMM were also here more often positive. In analysis of PD-L1 expression and overall survival (OS) they showed that patients with lower PD-L1 expression (using both 1% and 50% as a cut-offs) have better OS. However, after multivariate analyses, and adjusting for histology, performance status, smoking and treatment arm, this was no longer statistically significant. This raises the question whether PD-L1 positivity is just a surrogate marker for SMM, which could explain its association with worse prognosis.
Another important question is can PD-L1 expression in MM be used as a predictive biomarker for immunotherapy. Retrospective study by Rivalland (61) on a very small group of patients showed better objective response rate (ORR) was associated with higher PD-L1 expression. Applying cut-off of 5% for PD-L1 positivity, ORR was 40%, increasing to 50% when cut-off of 50% was applied. In PD-L1 negative patients, ORR was 22%. Although there are ongoing trials evaluating immunotherapy in a second and third line settings, with relation to PD-L1 expression, MAPS2 randomized non-comparative phase 2 trial showed improved objective response and disease control rates in patients expressing PD-L1 both with 1% and 25% used as a cut-off (62). In many clinical trials where there was obvious clinical benefit of immunotherapy, it was regardless of PD-L1 expression (63-66). Only one study of nivolumab and ipilimumab showed tendency of greater benefit for PD-L1 positive patients, however the study cohort was very small (only 34 patients). Like in other solid tumor types, there are many issues with PD-L1 testing: different antibody clones, which are similar but not the same, different cut-off values used in different studies, and tumoral and spatial heterogeneity. This results in different percentages of positive MM samples, ranging from 18% to 72% (54-59,67). Furthermore, it might also explain (lack of) correlation with the response to immunotherapy. Brosseau study also failed to demonstrate predictive significance of PD-L1 expression for bevacizumab-pemetrexed/cisplatin therapy. Interestingly, when analyzing only EMM patients, and using 50% as a cut-off for PD-L1 positivity, they showed a non-significant trend: patients with PD-L1 expression <50% demonstrated overall survival (OS) of 23 months in comparison to 12.3 months in a group where PD-L1 was present in 50% or more tumor cells. However, using the same cut-off for progression free survival (PFS) analyses, PD-L1 proved to be significant and independent prognostic factor (aHR 2.16; 95 CI, 1.2–3.84; P=0.0087); PD-L1 positive tumors showed 6.7 months of median PFS versus 9.9 months in low expression or negative group (60).
Another promising predictive biomarker is mesothelin, a membrane antigen, highly expressed in EMM and used as a target for new therapies (68,69). Drug-conjugated antibody against mesothelin, anetumab ravtansine, in a phase II study failed to show better progression free survival or OS in comparison to vinorelbin, as a second-line therapy (70). On the other hand, immunotoxin SS1P composed of anti-mesothelin antibody and pseudomonas exotoxin, in a phase I study, induced partial response in 77% of patients, although there were only 12 patients involved in this phase (71). Furthermore, application of a chimeric anti-mesothelin monoclonal antibody, amatuximab, with standard chemotherapy as a first line therapy, induced disease control rates of 90% (72). Innovative approach combining mesothelin expression and immunotherapy includes chimeric antigen receptor T cells (CAR-T) modified in a way to bind with tumor cells expressing mesothelin, and stimulating T cells to destroy them, and is currently in early phase clinical studies (73). Unfortunately, mesothelin is not expressed in SMM, so all mentioned above relates only to EMM. It seems that the SMM may respond to arginine deprivation therapy. Argininosuccinate synthetase 1 (ASS1) is the enzyme limiting arginine production, and is associated with increased tumorigenesis and more aggressive disease. In a phase I study, arginine depletion agent ADI‐PEG 20 (PEGylated arginine deiminase) was applied with standard-of-care chemotherapy in nine ASS1-deficient patients (proven by immunohistochemistry), among whom were 5 patients with MM (74). Seven patients demonstrated partial response, including 3 with SMM or BMM, and all of the patients had stable disease. Based on this results, patients with ASS1 loss in 75% of tumor were included in a phase II/III study whose results should be soon published (75)
It has been known that BAP-1 loss detected by immunohistochemistry is used as a proof of malignancy, and therefore in differentiation of reactive mesothelial proliferation versus MM (76-81). BAP1 is frequently lost in epithelioid diffuse MM in contrast to sarcomatoid mesothelioma. Furthermore, BAP1 loss induces enhancer of zeste homologue 2 (EZH2)-dependent transformation increasing trimethylated histone H3 lysine 27 (H3K27me3) and repressing polycomb repressive complex 2 (PRC2), inducing mesothelial proliferation, migration, and tumorigenesis (82,83) Inhibition of EZH2 in MM with BAP1 loss induced apoptosis and prevented tumor formation (82). EZH2 inhibitor, tazemetostat, is investigated in a phase II clinical study in MM patients (NCT02860286).
Conclusions
In this review, we have demonstrated well known diversity in histologic presentations of MMs that have prognostic significance and impact on treatment decisions. A correlation between morphology of malignant mesothelioma and predictive biomarkers is still in the development, and large clinical trials may give us the answers that would guide pathology practice and biomarker testing in this fatal disease.
Acknowledgments
Authors are grateful to Iva Brcic, MD, PhD, for her help in editing this manuscript and to Prof. Francoise Galateau Salle for providing image of malignant mesothelioma with transitional pattern.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.03.38). The series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” was commissioned by the editorial office without any sponsorship or funding. LB reports grants, personal fees and non-financial support from AstraZeneca, personal fees from Boehringer-Ingelheim, personal fees and non-financial support from MSD, personal fees from Takeda, personal fees and non-financial support from Roche, personal fees and non-financial support from Pfizer, personal fees from Eli Lilly, outside the submitted work. IK has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Robinson BWS, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [Crossref] [PubMed]
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v31-9. [Crossref] [PubMed]
- Liu B, van Gerwen M, Bonassi S, et al. Epidemiology of Environmental Exposure and Malignant Mesothelioma. J Thorac Oncol 2017;12:1031-45. [Crossref] [PubMed]
- Røe OD, Stella GM. Malignant pleural mesothelioma: history, controversy and future of a manmade epidemic. Eur Respir Rev 2015;24:115-31. [Crossref] [PubMed]
- Baumann F, Carbone M. Environmental risk of mesothelioma in the United States: An emerging concern-epidemiological issues. J Toxicol Environ Health B Crit Rev 2016;19:231-49. [Crossref] [PubMed]
- Opitz I, Friess M, Kestenholz P, et al. A New Prognostic Score Supporting Treatment Allocation for Multimodality Therapy for Malignant Pleural Mesothelioma: A Review of 12 Years' Experience. J Thorac Oncol 2015;10:1634-41. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results of 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [Crossref] [PubMed]
- Flores RM, Zakowski M, Venkatraman E, et al. Prognostic factors in the treatment of malignant pleural mesothelioma at a large tertiary referral center. J Thorac Oncol 2007;2:957-65. [Crossref] [PubMed]
- Bille A, Belcher E, Raubenheimer H, et al. Induction chemotherapy, extrapleural pneumonectomy, and adjuvant radiotherapy for malignant pleural mesothelioma: experience of Guy’s and St. Thomas’ hospitals. Gen Thorac Cardiovasc Surg 2012;60:289-96. [Crossref] [PubMed]
- Tracey E, Kerr T, Dobrovic A, et al. Cancer in NSW: Incidence and Mortality Report 2008. Cancer Institute NSW, Sydney, 2010.
- Neumann V, Günthe S, Mülle KM, et al. Malignant mesothelioma-German mesothelioma register 1987-1999. Int Arch Occup Environ Health 2001;74:383-95. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the International Association for the Study of Lung Cancer Mesothelioma database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Galateau-Salle F. Churg A. Roggli V.et al:Diffuse malignant mesothelioma. In:Travis WD, Brambilla E. Burke AP, Marx A. Nicholson AG (eds). WHO Classifiction of Tumours of the Lung, Pleura, Thymus and Heart. International Agency for Research on Cancer, Lyon, France, 2015;156-68.
- Kadota K, Suzuki K, Sima CS, et al. Pleomorphic epithelioid diffuse malignant pleural mesothelioma:a clinicopathological review and conceptual proposal to reclassify as biphasic or sarcomatoid mesothelioma. J Thorac Oncol 2011;6:896-904. [Crossref] [PubMed]
- Alchami FS., Attanoos RL., Bamber AR. Myxoid variant epithelioid pleural mesothelioma defines a favourable prognosis group: an analysis of 191 patients with pleural malignant mesothelioma. J Clin Pathol 2017;70:179-82. [Crossref] [PubMed]
- Brčić L., Jakopović M., Brčić I, et al. Reproducibility of histological subtyping of malignant pleural mesothelioma. Virchows Arch 2014;465:679-85. [Crossref] [PubMed]
- Galateau Salle F, Le Stang N, Nicholson AG, et al. New Insights on Diagnostic Reproducibility of Biphasic Mesotheliomas: A Multi-Institutional Evaluation by the International Mesothelioma Panel From the MESOPATH Reference Center. J Thorac Oncol 2018;13:1189-203. [Crossref] [PubMed]
- Vigneswaran WT, Kircheva DY, Ananthanarayanan V, et al. Amount of Epithelioid Differentiation Is a Predictor of Survival in Malignant Pleural Mesothelioma. Ann Thorac Surg 2017;103:962-6. [Crossref] [PubMed]
- Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol 2012;25:260-71. [Crossref] [PubMed]
- Rosen LE, Karrison T, Ananthanarayanan V, et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: a multi-institutional study. Mod Pathol 2018;31:598-606. [Crossref] [PubMed]
- Galateau-Sallé F, Vignaud JM, Burke L, et al. Well-differentiated papillary mesothelioma of the pleura:a series of 24 cases. Am J Surg Pathol 2004;28:534-40. [Crossref] [PubMed]
- Butnor KJ, Sporn TA, et al. Well-differentiated papillary mesothelioma. Am J Surg Pathol 2001;25:1304-9. [Crossref] [PubMed]
- Churg A, Allen T, Borczuk AC, et al. Well-differentiated papillary mesothelioma with invasive foci. Am J Surg Pathol 2014;38:990-8. [Crossref] [PubMed]
- Nemoto H, Tate G, Kishimoto K, et al. Heterozygous loss of NF2 is an early molecular alteration in well-differentiated papillary mesothelioma of the peritoneum. Cancer Genet 2012;205:594-8. [Crossref] [PubMed]
- Yu W, Chan-On W, Teo M, et al. First somatic mutation of E2F1 in a critical DNA binding residue discovered in well-differentiated papillary mesothelioma of the peritoneum. Genome Biol 2011;12:R96. [Crossref] [PubMed]
- Ribeiro C, Campelos S, Moura CS, et al. Well-differentiated papillary mesothelioma: clustering in a Portuguese family with a germline BAP1 mutation. Ann Oncol 2013;24:2147-50. [Crossref] [PubMed]
- Stevers M, Rabban JT, Garg K, et al. Well-differentiated papillary mesothelioma of the peritoneum is genetically defined by mutually exclusive mutations in TRAF7 and CDC42. Mod Pathol 2019;32:88-99. [Crossref] [PubMed]
- Allen TC, Cagle PT, Churg AM, et al. Localized malignant mesothelioma. Am J Surg Pathol 2005;29:866-73. [Crossref] [PubMed]
- Crotty TB, Myers JL, Katzenstein AL, et al. Localized malignant mesothelioma. A clinicopathologic and flow cytometric study. Am J Surg Pathol 1994;18:357-63. [Crossref] [PubMed]
- Hung YP, Dong F, Dubuc AM, et al. Molecular characterization of localized pleural mesothelioma. Mod Pathol 2020;33:271-80. [Crossref] [PubMed]
- Marchevsky AM, Khoor A, Walts AE, et al. Localized malignant mesothelioma, an unusual and poorly characterized neoplasm of serosal origin: best current evidence from the literature and the International Mesothelioma Panel. Mod Pathol 2020;33:281-96. [Crossref] [PubMed]
- Ordóñez NG. Pleomorphic mesothelioma: report of 10 cases. Mod Pathol 2012;25:1011-22. [Crossref] [PubMed]
- Hammar SP, Henderson DW, Klebe S, et al. Neoplasmas of pleura. In: Tomashefski JF Jr. editor. Dail and Hammar’s pulmonary pathology. 3rd ed. Vol 2. Springer, New York, 2008:558-734.
- Dacic S, Le Stang N, Husain A, et al. Interobserver variation in the assessment of the sarcomatoid and transitional components in biphasic. Mod Pathol 2020;33:255-62. [Crossref] [PubMed]
- Henderson DW, Attwood HD, Constance TJ, et al. Lymphohistiocytoid mesothelioma: a rare lymphomatoid variant of predominantly sarcomatoid mesothelioma. Ultrastruct Pathol 1988;12:367-84. [Crossref] [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma:2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Galateau-Sallé F, Attanoos R, Gibbs AR, et al. Lymphohistiocytoid variant of malignant mesothelioma of the pleura: a series of 22 cases. Am J Surg Pathol 2007;31:711-6. [Crossref] [PubMed]
- Talerman A, Montero JR, Chilcote RR, et al. Diffuse malignant peritoneal mesothelioma in a 13-year-old girl. Report of a case and review of the literature. Am J Surg Pathol 1985;9:73-80. [Crossref] [PubMed]
- Paliogiannis P, Putzu C, Ginesu GC, et al. Deciduoid mesothelioma of the thorax: A comprehensive review of the scientific literature. Clin Respir J 2018;12:848-56. [Crossref] [PubMed]
- Mayall FG, Gibbs AR. The histology and immunohistochemistry of small cell mesothelioma. Histopathology 1992;20:47-51. [Crossref] [PubMed]
- Ordóñez NG. Mesotheliomas with small cell features: report of eight cases. Mod Pathol 2012;25:689-98. [Crossref] [PubMed]
- Wang H, Herath C. Signet ring cell mesothelioma; A diagnostic challenge. Pathol Res Pract 2019;215:152462. [Crossref] [PubMed]
- Ordóñez NG. Mesothelioma with signet-ring cell features: report of 23 cases. Mod Pathol 2013;26:370-84. [Crossref] [PubMed]
- Nicholson AG, Sauter JL, Nowak AK, et al. EURACAN/IASLC Proposals for Updating the Histologic Classification of Pleural Mesothelioma: Towards a More Multidisciplinary Approach. J Thorac Oncol 2020;15:29-49. [Crossref] [PubMed]
- Cantin R, Al-Jabi M, McCaughey WT. Desmoplastic diffuse mesothelioma. Am J Surg Pathol 1982;6:215-22. [Crossref] [PubMed]
- Berg KB, Churg A. GATA3 Immunohistochemistry for Distinguishing Sarcomatoid and Desmoplastic Mesothelioma From Sarcomatoid Carcinoma of the Lung. Am J Surg Pathol 2017;41:1221-5. [Crossref] [PubMed]
- Klebe S, Mahar A, Henderson DW, et al. Malignant mesothelioma with heterologous elements: clinicopathological correlation of 27 cases and literature review. Mod Pathol 2008;21:1084-94. [Crossref] [PubMed]
- Kiyozuka Y, Miyazaki H, Yoshizawa K, et al. An autopsy case of malignant mesothelioma with osseous and cartilaginous differentiation: bone morphogenetic protein-2 in mesothelial cells and its tumor. Dig Dis Sci 1999;44:1626-31. [Crossref] [PubMed]
- Demirag F, Unsal E, Tastepe I. Biphasic malignant mesothelioma cases with osseous differentiation and long survival: a review of the literature. Lung Cancer 2007;57:233-6. [Crossref] [PubMed]
- Klebe S, Brownlee NA, Mahar A, et al. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010;23:470-9. [Crossref] [PubMed]
- Hashimoto K, Okuma Y, Hosomi Y, et al. Malignant mesothelioma of the pleura with desmoplastic histology: a case series and literature review. BMC Cancer 2016;16:718. [Crossref] [PubMed]
- Kao SC, Yan TD, Lee K, et al. Accuracy of diagnostic biopsy for the histological subtype of malignant pleural mesothelioma. J Thorac Oncol 2011;6:602-5. [Crossref] [PubMed]
- Chirieac LR, Hung YP, Foo WC, et al. Diagnostic value of biopsy sampling in predicting histology in patients with diffuse malignant pleural mesothelioma. Cancer 2019;125:4164-71. [Crossref] [PubMed]
- Cedrés S, Ponce-Aix S, Zugazagoitia J. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015;10:e0121071. [Crossref] [PubMed]
- Kao SC, Cheng YY, Williams M, et al. Tumor Suppressor microRNAs Contribute to the regulation of PD-L1 Expression in Malignant Pleural Mesothelioma J Thorac Oncol 2017;12:1421-33. [published correction appears in J Thorac Oncol 2018;13:587]. [Crossref] [PubMed]
- Thapa B, Salcedo A, Lin X, et al. The Immune Microenvironment, Genome-wide Copy Number Aberrations, and Survival in Mesothelioma. J Thorac Oncol 2017;12:850-9. [Crossref] [PubMed]
- Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 2014;9:1036-40. [Crossref] [PubMed]
- Nguyen BH, Montgomery R, Fadia M, et al. PD-L1 expression associated with worse survival outcome in malignant pleural mesothelioma. Asia Pac J Clin Oncol 2018;14:69-73. [Crossref] [PubMed]
- Combaz-Lair C, Galateau-Sallé F, McLeer-Florin A, et al. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum Pathol 2016;52:9-18. [Crossref] [PubMed]
- Brosseau S, Danel C, Scherpereel A, et al. Shorter Survival in Malignant Pleural Mesothelioma Patients With High PD-L1 Expression Associated With Sarcomatoid or Biphasic Histology Subtype: A Series of 214 Cases From the Bio-MAPS Cohort. Clin Lung Cancer 2019;20:e564-75. [Crossref] [PubMed]
- Rivalland G, Kao SCH, Pavlakis N, et al. Outcomes of anti-PD-1 therapy in mesothelioma and correlation with PD-L1 expression. J Clin Oncol 2017;35:8514. [Crossref]
- Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019;20:239-53. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Quispel-Janssen J, van der Noort V, de Vries JF, et al. Programmed Death 1 Blockade With Nivolumab in Patients With Recurrent Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1569-76. [Crossref] [PubMed]
- Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1):an open-label, non-randomised, phase 2 study. Lancet Respir Med 2018;6:451-60. [Crossref] [PubMed]
- Lee HS, Jang HJ, Choi JM, et al. Comprehensive immunoproteogenomic analyses of malignant pleural mesothelioma. JCI Insight 2018;3:7. [Crossref] [PubMed]
- Chapel DB, Stewart R, Furtado LV, et al. Tumor PD-L1 expression in malignant pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and Dako PD-L1 28-8 pharmDx assays. Hum Pathol 2019;87:11-7. [Crossref] [PubMed]
- Inaguma S, Wang Z, Lasota J, et al. Comprehensive immunohistochemical study of mesothelin (MSLN) using different monoclonal antibodies 5B2 and MN-1 in 1562 tumors with evaluation of its prognostic value in malignant pleural mesothelioma. Oncotarget 2017;8:26744-54. [Crossref] [PubMed]
- Eguchi T, Kadota K, Mayor M, et al. Cancer antigen profiling for malignant pleural mesothelioma immunotherapy: expression and coexpression of mesothelin, cancer antigen 125, and Wilms tumor 1. Oncotarget 2017;8:77872-82. [Crossref] [PubMed]
- Kindler HL, Novello S, Fennell D, et al. OA 02.01 Randomized Phase II Study of Anetumab Ravtansine or Vinorelbine in Patients with Metastatic Pleural Mesothelioma. J Thorac Oncol 2017;12:S1746. [Crossref]
- Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer 2014;120:3311-9. [Crossref] [PubMed]
- Hassan R, Kindler HL, Jahan T, et al. Phase II clinical trial of amatuximab, a chimeric antimesothelin antibody with pemetrexed and cisplatin in advanced unresectable pleural mesothelioma. Clin Cancer Res 2014;20:5927-36. [Crossref] [PubMed]
- Hassan R, Thomas A, Alewine C, et al. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J Clin Oncol 2016;34:4171-9. [Crossref] [PubMed]
- Beddowes E, Spicer J, Chan PY, et al. Phase 1 Dose-Escalation Study of Pegylated Arginine Deiminase, Cisplatin, and Pemetrexed in Patients With Argininosuccinate Synthetase 1-Deficient Thoracic Cancers. J Clin Oncol 2017;35:1778-85. [Crossref] [PubMed]
- Szlosarek PW, Baas P, Ceresoli GL, et al. ATOMIC-Meso: A randomized phase 2/3 trial of ADI- PEG20 or placebo with pemetrexed and cisplatin in patients with argininosuccinate synthetase 1- deficient non-epithelioid mesothelioma. J Clin Oncol 2017;35:abstr TPS8582.
- Yoshikawa Y, Sato A, Tsujimura T, et al. Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci 2012;103:868-74. [Crossref] [PubMed]
- Hwang HC, Pyott S, Rodriguez S, et al. BAP1 immunohistochemistry and p16 FISH in the diagnosis of sarcomatous and desmoplastic mesotheliomas. Am J Surg Pathol 2016;40:714-8. [Crossref] [PubMed]
- Churg A, Sheffield BS, Galateau-Salle F. New markers for separating benign from malignant mesothelial proliferations: are we there yet? Arch Pathol Lab Med 2016;140:318-21. [Crossref] [PubMed]
- Sheffield BS, Hwang HC, Lee AF, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977-82. [Crossref] [PubMed]
- Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015;28:1043-57. [Crossref] [PubMed]
- Hida T, Hamasaki M, Matsumoto S, et al. BAP1 immunohistochemistry and p16 FISH results in combination provide higher confidence in malignant pleural mesothelioma diagnosis: ROC analysis of the two tests. Pathol Int 2016;66:563-70. [Crossref] [PubMed]
- LaFave LM, Béguelin W, Koche R, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med 2015;21:1344-9. [Crossref] [PubMed]
- Bononi A, Giorgi C, Patergnani S, et al. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature 2017;546:549-53. [Crossref] [PubMed]


