
Managing screening-detected subsolid nodules—the Asian perspective
Introduction
The widely adopted use of computed tomography (CT) for lung cancer screening has led to detection of large numbers of pulmonary nodules that require further evaluation and management. Among the detected nodules, those with a radiological ground glass pattern are classified as subsolid or ground glass nodules (GGNs), which include both non-solid nodules (pure GGNs) and part-solid nodules (mixed GGNs). In Asia, many GGNs are detected by screening that is either specifically targeted to find them or done for other medical purposes, particularly among female non-smokers and those with a family history of lung cancer (1,2). Because of their reportedly high probability of being lung adenocarcinoma or premalignant lesions (3), detected GGNs tend to be directly referred to thoracic surgeons for surgical evaluation. Although the growth of GGNs is generally slow or even indolent, some grow rapidly during follow-up (4), which often makes it challenging to make a decision regarding the management of these nodules (5-7).
To facilitate a more concrete discussion, we present three typical clinical case scenarios that highlight particularly important issues: a small pure GGN the resection of which is strongly desired by the patient against the guidelines; a centrally located GGN; and multifocal GGNs.
Clinical scenarios
Case 1
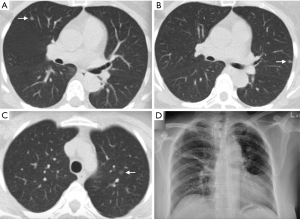
A 50-year-old male banker received an industry-provided health examination. A subcentimeter peripheral GGN was found in the right upper lobe (RUL) on chest CT (Figure 1A), and it remained stable on the follow-up CT 6 months later. Continuous long-term follow-up until the lesion size increased or a solid component emerged was initially recommended; however, the patient insisted on receiving surgical resection after considering the risk of minimally invasive surgery and the impact of pulmonary functional loss after a simple wedge resection, although the size and the growth pattern of the GGN did not yet meet the criteria to mandate resection. The patient received thoracoscopic wedge resection 7 months after detection, and the pathology reported minimally invasive adenocarcinoma. The cost of surgery and hospitalization was totally covered by the national health insurance system.

Case 2
A 35-year-old female office worker who worked in a hospital received the annual health-examination in her workplace. A 1 cm centrally located GGN was found in the RUL (Figure 1B), and the GGN became heterogeneous during the 2 years of imaging follow-up. However, she hesitated to undergo lobectomy directly without tissue diagnosis of the lesion, and she instead went to another hospital to receive semi-anatomical segmentectomy under the guidance of lesion localization with a fiducial marker (microcoil). The pathology report indicated minimally invasive adenocarcinoma and the resection margin was sufficient.
Case 3
A 58-year-old female, who had retired from work, received low-dose CT screening in a medical center. Four small lung nodules were detected bilaterally and the doctor suggested follow-up with low-dose CT. However, she visited several hospitals for second opinions during the 1-year follow-up period. She received a positron emission tomography (PET) scan in one of the hospitals, and it showed three nodules with higher standardized uptake value (SUV) and no evidence of nodal metastasis. She finally underwent thoracoscopic RUL anterior segmentectomy for the RUL lesion (Figure 2A), and one month later, thoracoscopic left upper lobe (LUL) wedge resection for another peripheral LUL lesion (Figure 2B) with apical trisegmentectomy for the central LUL lesion (Figure 2C). The pathology report indicated primary lung adenocarcinomas for all the three lesions, and the lymph nodes from both sides were all negative. A chest X-ray taken 3 months after surgery is shown in Figure 2D. Her functional status remained excellent during the 2 years of follow-up after surgery.
Challenges of guidelines
There may or may not be clinical guidelines on the management of pulmonary nodules in Asian countries and where guidelines are provided, they vary widely. There are national society guidelines in Japan (8), Korea (9) and China, and in other countries healthcare practitioners optionally refer to individual institutional standards or to western guidelines. To provide common guidelines for practitioners throughout Asia, consensus-based expert recommendations were used to produce a set of consensus guidelines by adapting the guidelines of the American College of Chest Physicians (CHEST) (1). However, the above guidelines do not seem to be utilized commonly in practice, and most clinicians tend to manage those detected nodules according to their own experience in the interpretation of CT images and the personality of individual patients, even in countries with national society guidelines. Ideally, every patient should be discussed at a multidisciplinary team meeting (MDT) to determine optimal diagnostic and therapeutic strategy. In general, the guidelines may often not be utilized for several reasons: (I) national health insurance covers the medical expenses of all treatments for screened nodules, including those outside the regulations of the existing guidelines, causing ultimate clinical decisions to be readily affected by either the physician’s experience or sometimes the patient’s own will. (II) In Asia, many institutes have in actuality adopted the concept of shared decision making (SDM) with patients in the management of screened nodules, although this is not formally documented. SDM has, in fact, been adopted in many western medical systems for lung cancer screening (10-12), including in ongoing randomized trials (13). Because of anxiety, some patients prefer to have the small GGNs removed as long as there is some probability of malignancy. The cost and radiation exposure involved in long-term monitoring of GGNs is another cause of concern leading to premature removal. (III) Many specialists with different backgrounds, including pulmonology, radiology, thoracic surgery, and family medicine, are involved in the management of small GGNs. However, different specialists and their associated medical societies might not adopt other society’s guidelines, or guidelines may not exist in their own society.
Role of preoperative biopsies and imaging work-ups
Increased experience in managing small GGNs has led to a great decline in the importance of preoperative biopsy. Although nonsurgical biopsy still remains the standard diagnostic option for a suspicious nodule, many thoracic surgeons prefer direct surgical resection for diagnosis and treatment. The main reason in support of this practice is a better understanding of the correlation between CT characteristics and histopathological findings (14-16), which has prompted surgical removal of these small GGNs on the basis of radiological diagnosis. Although a high specificity (0.94; 95% CI, 0.84–0.98) and sensitivity (0.92; 95% CI, 0.88–0.95) of percutaneous CT-guided biopsy for GGNs has been reported in meta-analyses (17,18), the diagnostic accuracy of small-sized (<1 cm) GGNs with a lower percentage of ground glass component tended to be lower, which made thoracic surgeons reluctant to perform preoperative biopsy for these small-sized GGNs. Additionally, the risk of complications, such as pneumothorax, hemoptysis and air embolism, is also a reason against biopsy. Compared with percutaneous CT-guided biopsy, the complication rate of transbronchial biopsy is relatively lower, but the reported diagnostic rate is also lower than CT-guided biopsy in general (19-21). However, a negative biopsy still does not totally preclude the possibility of malignancy. Thoracic surgeons may feel the necessity of preoperative biopsy for centrally located lesions in which pulmonary lobectomy is required for resection of the lesion, and tissue diagnosis might be needed to justify such a major surgical procedure.
When treating lung cancers, complete staging with a brain and whole-body imaging workup is considered mandatory in current clinical practice (22,23). An imaging workup using brain MRI in combination with whole body PET/CT scan is the first choice to detect possible distant metastasis of lung cancer. However, to save cost and time, a brain CT and whole-body bone scan is an optional alternative in some institutes, although this combination is less sensitive in detecting metastasis. On the basis of wanting a complete imaging workup for lung cancer, most countries in Asia adopt the more sensitive imaging policy for suspicious GGNs before undertaking surgery. However, the value of routine imaging workups for the GGNs is still questionable and sometimes considered unnecessary (24) because there is very little chance of distant metastasis, especially for pure GGNs (25). Interestingly, sometimes incidental findings on these PET/CT scans include second primary cancers of other organs, but this might not be able to justify the routine use of such advanced imaging modalities. In our opinion, it is quite reasonable to adopt these stringent preoperative imaging workups for the part-solid GGNs, which are considered relatively invasive with higher probability of nodal involvement compared with pure GGNs; however, the debate on differentiating benign and malignant GGNs with PET scans goes beyond the discussion of complete staging before surgery (26,27).
Location of GGNs: what really matters
The current guidelines for GGN management mainly focus on the size, solidity, and growth rate of the nodules (1,8,9). However, the location of the nodules, which is less mentioned in the guidelines, is critical in decision making in real clinical practice. Because the technical difficulty and the loss of lung tissue for acquiring adequate resection margins varies depending on the location of small GGNs, clinicians should always think of the potential risks and benefits for individual patients, especially when the benefit of surgical resection for small GGNs remains controversial. For example, when treating a small peripheral pure GGN such as the one shown in Figure 1A, a simple wedge resection by thoracoscopic surgery would be an easy decision because of the low technical demand for a surgeon and also minimal loss of lung tissue for the patient. However, if a similar lesion is located centrally as shown in Figure 1B, the decision would be more difficult, especially for elderly patients with limited function and/or limited expected prognosis. Indeed, some thoracic surgeons tend to continue observation of such central lesions until pulmonary lobectomy is strongly indicated. Alternatively, if sublobar resection is attempted, there should be enough confidence of success in complete resection with adequate margin for the target lesion. For the resection of central lesions, obtaining sufficient resection margins with a direct wedge resection appears challenging. Conventional anatomical segmentectomy is also sometimes inadequate because the lesion is located too close to an adjacent segment or even between two segments, which necessitates various types of segmentectomy to secure the resection margins—including extended segmentectomy, semi-anatomical segmentectomy, or combined subsegmentectomy—and the determination of the appropriate resection plane between segments or subsegments is critical (28).
Localization: beyond what we think for sublobar resection
For surgical management of small subsolid nodules, preoperative localization is important when performing sublobar lung resection. In addition to localization of the targeted tumor, acquisition of sufficient resection margins is critical to prevent local recurrence (29-31). The conventional marking strategy is to place a single marker on the lung surface, which is effective especially when the target lesion is located near the surface. Most CT-guided percutaneous localization utilizes a single surface marking strategy, and this is probably the most commonly used localization method; however, with this method there is still concern about localization-related complications (32,33) including pneumothorax, pulmonary hemorrhage and most importantly air embolism, which is rare but critical (34-36). Because of the above concerns, the authors prefer to use a transbronchial localization method, which has been demonstrated to be relatively safe with no reported fatal complications. Transbronchial localization can be guided by virtual bronchoscopy software (37), an electromagnetic bronchoscopy system (38,39) and a cone-beam CT with augmented fluoroscopy (40,41). Additionally, multiple surface markings can be made easily with the transbronchial approach. Compared with conventional point-directed single-dimensional localization, multiple surface markings, which can be referred to as “lung mapping,” can provide two-dimensional geometric information for better control of the resection border, and the system of virtual-assisted lung mapping (VAL-MAP) has already been widely adopt and covered by public health insurance in Japan as one of the main approaches to localize small GGNs (42). However, for some deeply-seated GGNs, even two-dimensional localization might not guarantee sufficient resection margins at greater depths (43), and the concept of three-dimensional localization or mapping with centrally placed fiducial markers has been raised for acquiring adequate deep margins in a standardized and reproducible manner. Therefore, a next-generation lung mapping system (VAL-MAP 2.0) has been developed (44), and a phase III prospective trial is also in progress to evaluate the effectiveness of small-nodule resections with optimal resection margins (45). Because the new technologies and modalities for localization will continue to progress, surgeons in different institutions should choose a suitable method, based on the facilities they can use, while balancing efficacy, safety, and cost. The priorities for adopting new localization technology should be the reproducibility of surgery and the acquisition of resection margins.
Intra-operative frozen section: when is it needed?
Varoli et al. (46) reported their surgical experience [1991–2006] of performing resections of 370 solitary pulmonary nodules without preoperative diagnosis. Frozen sections were performed on all the 276 wedge resections with nodules included, while the other patients received lobectomy directly because of the difficulty of wedge resection. When the frozen pathology showed primary lung cancer, the procedure was converted to lobectomy in the same session. In the modern era of numerous small nodules, frozen sections are still routinely performed in many centers. However, now that lobectomy as the standard final procedure for lung cancer has been greatly replaced by sublobar resection, the practice of routinely performing frozen sections on indeterminate lung nodules has raised some questions. Regarding the role of intraoperative frozen section, the possibility of having to alter the final surgical plan was the only reason to justify this urgent examination. In the following situations, frozen section analysis could be necessary for intraoperative decision making: (I) when a further planned resection—lobectomy, segmentectomy, or additional wedge—would need to be completed if malignancy is confirmed; (II) to evaluate the existence of a high-grade invasive component, which might also change the resection plan, although the validity of such a decision remains controversial (47,48); (III) to confirm that the target lesion was successfully resected when success cannot be judged by gross examination, especially for small GGNs; (IV) when the surgical procedure would be extended to lymphadenectomy if malignancy of the lesion is confirmed. In the current practice, most surgical procedures are decided prior to surgery according to the findings of CT images, although many current series still report that frozen section is routinely performed without changing the subsequent surgical procedures. The critical point in real clinical practice is: should the diagnosis be made right after the surgery, even when there is no chance of changing the surgical plan? If the answer is negative, it may be preferable to save the specimen for a permanent section, which could provide more comprehensive pathological evaluation of the specimen (49).
Mediastinal lymph node (MLN) dissection, sampling, or omitting assessment?
Lymph node staging was long considered mandatory for lung cancer, and surgical exploration of MLN nodes was usually performed as a standard procedure of lung cancer surgery. However, more thoracic surgeons are now adopting a less aggressive manner of MLN dissection when doing surgery for part-solid nodules because of the rarity of lymph node metastasis (50). Also, there is still risk of complications, including hoarseness, chylothorax, and injury to adjacent structures (51,52). For a pure GGN, although there is still up to a 40% probability of the final diagnosis being invasive adenocarcinoma (53), most current literature has reported negative nodal involvement (50,54,55) even when the lesion size exceeds 3 cm (56). Moon et al. (57) reported 358 cases of clinical nodal negative (N0) GGNs (<3 cm in size), which received either MLN dissection, MLN sampling or no MLN assessment. In 129 GGNs with consolidation/tumor (C/T) ratio <0.5, only one case had intralobar (N1) nodal metastasis. For the other 229 GGNs with C/T ratio >0.5, twenty-five cases had nodal upstaging after resection. Among such consolidation-dominant GGNs, although the 5-year recurrence free survival was comparable in patients receiving MLN sampling and MLN dissection, the recurrence free survival was significantly poorer in the group where MLN assessment was omitted. In another cohort with cT1N0 peripheral lung cancers undergoing lobectomy and MLN dissection, nodal upstaging was observed among nodules with C/T ratio >0.61 or size >1.3 cm (58). Aside from lymph node sampling, selective or lobar-specific lymph node dissection has also been reported as an effective alternative to systemic dissection (59-61). In our current opinion, for pure-GGNs, omitting MLN assessment is a reasonable option. For part-solid GGNs, MLN sampling or selective MLN dissection could be an alternative to systemic MLN dissection; however, there is still no consensus on this issue.
Management of multifocal subsolid nodules
Among patients with screened nodules, there are many who present with synchronous multifocal subsolid nodules, which are considered to be separate primary lesions instead of intrapulmonary metastasis (62). The patient shown in Figure 2 is a typical example. In current series of surgically managed early stage lung cancers, similar multifocal lesions have been found in up to 5–20% of all patients (63-66). Although the long-term survival of these patients is usually favorable, management of these multifocal lesions with different characteristics, sizes and locations is challenging due to the lack of consensus or established algorithms, which results in clinical judgement being almost totally dependent on the surgeons’ own experience. To exclude the possibility of extrapulmonary and nodal metastasis, PET/CT is the recommended examination for multiple lung lesions according to the National Comprehensive Cancer Network (NCCN) guidelines (67). Additionally, the 18F-fluorodeoxyglucose (FDG) uptake in PET scans provides information associated with the aggressiveness of each lesion (68-71), indicating the priority of surgical intervention, although it is considered less useful to perform PET scans for multifocal pure GGNs (66). In current practice, according to recent reports, the most common strategy is to prioritize treatment of the dominant lesion, defined by its size and radiological invasiveness, because it is considered to have the most effect on patient survival (64), and any residual GGNs with the risk of progression should not rule out the resection of the dominant lesion (72). Neither the growth nor the need for subsequent intervention for residual GGNs, influenced patient survival (72), strongly suggesting that these multifocal lesions should be treated in a separate, sequential manner with staged interventions during close, long-term observation (73). At the planning of such staged interventions, laterality of lesions should also be taken into consideration. For example, if the prioritized lesion accompanies a small pure GGN on the same side and it could be easily resected, concurrent resection is an option rather than waiting for a third operation (which would likely be accompanied by pleural and possibly hilar adhesions) following the second surgery on the other side for the second-dominant lesion. In the authors’ opinion, preserving the patient’s lung function and quality of life after surgery is most important. In addition to image surveillance for non-dominant lesions, comprehensive preoperative planning for each patient that includes precise localization, a lung-preserving surgical strategy, anatomy of the remaining lung, and estimation of residual lung function (74) is necessary to keep the balance between the resection of suspicious nodules and the preservation of the patient’s lung function.
Nonsurgical management of subsolid nodules
If patients are not amenable to surgery or if surgical resection seems unjustified in terms of the balance among functional loss, invasiveness, and radicality, non-surgical treatments such as stereotactic body radiotherapy (SBRT) and other local ablation therapies can be adopted as alternatives with curative intent for suspicious nodules. Particularly in the case of multifocal GGNs, combining surgery with such nonsurgical management is also a viable option. A major drawback of this approach is that in many cases the pathological diagnosis cannot be made without biopsy.
SBRT
SBRT typically involves delivery of a steep dose gradient beyond the small target while simultaneously avoiding the surrounding normal tissue. This technique relies on technological advances in image-guided radiation therapy to visualize the tumor both before and during treatment delivery, as well as to monitor respiratory motion (75). Currently, the NCCN and the European Society for Medical Oncology (ESMO) guidelines consider SBRT as the first-line non-surgical treatment option for medically inoperable patients with stage I non-small cell lung cancer (NSCLC). Population-based analyses (76,77) have demonstrated an improvement in overall survival following the introduction of SBRT in clinical practice for elderly patients with stage I NSCLC, and large retrospective observational studies have also confirmed the promising results of SBRT (78-80). Several research groups conducted phase I-II trials of SBRT for inoperable early-stage NSCLC, with 2–3-year local control (LC) rates and 1–3-year overall survival rates ranging between 84–98% and 43–72%, respectively (81). The role of SBRT for medically operable patients is yet to be determined and concerns remain about the risk of local or nodal recurrence after SBRT. Several population-based and retrospective analyses suggested that overall survival and disease-specific survival are similar compared to surgery (82-87); however, a recent phase II trial reported that the pathological complete response rate of early stage lung cancer after SBRT was only 60%, which was lower than expected (88). Although SBRT toxicity is generally mild, the risk of skin and rib toxicity when treating peripheral tumors and the risk of severe complications when treating central tumors are still issues of concern, and the optimal fractionation scheme for safe and effective SBRT delivery is under evaluation. The need for accurate nodal staging and pathological information is still challenging, and difficulties remain in the interpretation of radiological findings after SBRT, which appear similar in regard to local relapse and radiation-induced changes (75).
Radiofrequency ablation (RFA)
RFA is a common technique for the ablation of solid organs and also a relatively new treatment option for medically inoperable primary lung cancer. Advantages of RFA compared to surgery include treatment in an outpatient setting and the use of local anesthesia before placement of the ablation probe via CT guidance. Limitations of RFA include: (I) lesions greater than 3 cm in diameter are not recommended for RFA due to poor LC. (II) A heat sink effect occurs when tumors are located in close proximity to large vessels (>3 mm), reducing the energy delivered to the target through convection within the circulatory system. (III) Location is critical as a result of the risk of damage to adjacent structures, such as the esophagus and trachea (89,90). Several studies have examined the results of RFA as definitive therapy for early stage NSCLC (91-93). However, RFA has generally been associated with inferior LC compared to surgery and SBRT, with a 3-year LC rate of approximately 80% to 95% (94). Trials that are designed to evaluate RFA use in high-risk patients, such as ACOSOG Z4033, will help determine the indications for its use.
Microwave ablation (MWA)
MWA has theoretical advantages over RFA in treatment of the lung because microwaves are less prone to the heat sink effect and able to penetrate deeper into low-conductivity tissue such as lung parenchyma (95). Current applications of MWA in pulmonary lesions mostly involve metastatic lesions, and reported LC rates are comparable with SBRT without major adverse events (96-98). Interestingly, cancer-specific mortality is reportedly not significantly affected by tumor size larger than 3 cm, which may attribute to increased intratumoral temperatures with a larger ablation zone in MWA compared to RFA (94). However, limited data are available to support the use of MWA in the treatment of suspicious subsolid nodules.
Conclusions
Because of their high probability of being lung cancer, surgical resection of screening-detected subsolid nodules is the common practice in Asia. As clinical judgement is largely affected by the physician’s experience and the patient’s own will, the current treatment guidelines might frequently not be followed. However, guidelines continue to evolve and may more closely match actual clinical practice in the near future. With a better understanding of the oncological characteristics of these subsolid nodules, including patterns of disease progression, surgery will be performed in the manner of early intervention, reducing the need for whole-body scans. Such surgical intervention is likely to comprise a less extensive lymph node exploration and limited resection with increased lung preservation. To ensure the adequacy of performing sublobar resection with sufficient safety margins, precise localization plays an essential role, not only for the lesion itself, but also for the resection borders and deep margins. We believe it is most important for thoracic surgeons to keep a balance between surgical and oncological achievement and preservation of the patient’s lung function and quality of life after surgery. This is especially true for the management of multifocal nodules.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Paul Van Schil and Annemiek Snoeckx) for the series “Lung cancer screening” published in Translational Lung Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-243). The series “Lung cancer screening” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bai C, Choi CM, Chu CM, et al. Evaluation of Pulmonary Nodules Clinical Practice Consensus Guidelines for Asia. Chest 2016;150:877-93. [Crossref] [PubMed]
- Wu FZ, Huang YL, Wu CC, et al. Assessment of Selection Criteria for Low-Dose Lung Screening CT Among Asian Ethnic Groups in Taiwan: From Mass Screening to Specific Risk-Based Screening for Non-Smoker Lung Cancer. Clin Lung Cancer 2016;17:e45-e56. [Crossref] [PubMed]
- Migliore M, Fornito M, Palazzolo M, et al. Ground glass opacities management in the lung cancer screening era. Ann Transl Med 2018;6:90. [Crossref] [PubMed]
- Lee SW, Leem CS, Kim TJ, et al. The long-term course of ground-glass opacities detected on thin-section computed tomography. Respir Med 2013;107:904-10. [Crossref] [PubMed]
- Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:108S-130S.
- Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 2012;185:363-72. [Crossref] [PubMed]
- Zhan P, Xie H, Xu C, et al. Management strategy of solitary pulmonary nodules. J Thorac Dis 2013;5:824-9. [PubMed]
- Guidelines for the Management of Pulmonary Nodules Detected by Low-dose CT Lung Cancer Screening, Version 3. Available online: http://www.jscts.org/pdf/guideline/gls3rd_english130621.pdf
- Lee HJ, Kim JH, Kim YK, et al. Korean Society of Thoracic Radiology Guideline for Lung Cancer Screening with Low-Dose CT. J Korean Soc Radiol 2012;67:349-65. [Crossref]
- Lowenstein LM, Deyter GMR, Nishi S, et al. Shared decision-making conversations and smoking cessation interventions: Critical components of low-dose CT lung cancer screening programs. Transl Lung Cancer Res 2018;7:254-71. [Crossref] [PubMed]
- Brenner AT, Malo TL, Margolis M, et al. Evaluating Shared Decision Making for Lung Cancer Screening. JAMA Intern Med 2018;178:1311-6. [Crossref] [PubMed]
- Melzer AC, Golden SE, Ono SS, et al. What Exactly Is Shared Decision-Making? A Qualitative Study of Shared Decision-Making in Lung Cancer Screening. J Gen Intern Med 2020;35:546-53. [Crossref] [PubMed]
- Ruparel M, Quaife SL, Ghimire B, et al. Impact of a lung cancer screening information film on informed decision-making: A randomized trial. Ann Am Thorac Soc 2019;16:744-51. [Crossref] [PubMed]
- Saito H, Kameda Y, Masui K, et al. Correlations between thin-section CT findings, histopathological and clinical findings of small pulmonary adenocarcinomas. Lung Cancer 2011;71:137-43. [Crossref] [PubMed]
- Lee HY, Choi Y, La , Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: Histopathology, imaging, management. AJR Am J Roentgenol 2014;202:W224-33 [Crossref] [PubMed]
- Qi L, Xue K, Li C, et al. Analysis of CT morphologic features and attenuation for differentiating among transient lesions, atypical adenomatous hyperplasia, adenocarcinoma in situ, minimally invasive and invasive adenocarcinoma presenting as pure ground-glass nodules. Sci Rep 2019;9:14586. [Crossref] [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [Crossref] [PubMed]
- Yang JS, Liu YM, Mao YM, et al. Meta-analysis of CT-guided transthoracic needle biopsy for the evaluation of the ground-glass opacity pulmonary lesions. Br J Radiol 2014;87:20140276 [Crossref] [PubMed]
- Han Y, Kim H, Kong K, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: A systematic review and meta-analysis. PLoS One 2018;13:e0191590 [Crossref] [PubMed]
- Wang C, Li X, Zhou Z, et al. Endobronchial ultrasonography with guide sheath versus computed tomography guided transthoracic needle biopsy for peripheral pulmonary lesions: A propensity score matched analysis. J Thorac Dis 2016;8:2758-64. [Crossref] [PubMed]
- Zhan P, Zhu Q, Miu Y, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2017;6:23-34. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Lee HY, Lee KS, Kim BT, et al. Diagnostic efficacy of PET/CT plus brain MR imaging for detection of extrathoracic metastases in patients with lung adenocarcinoma. J Korean Med Sci 2009;24:1132-8. [Crossref] [PubMed]
- Song JU, Song J, Lee KJ, et al. Are there any additional benefits to performing positron emission tomography/computed tomography scans and brain magnetic resonance imaging on patients with ground-glass nodules prior to surgery? Tuberc Respir Dis (Seoul) 2017;80:368-76. [Crossref] [PubMed]
- Cho H, Lee HY, Kim J, et al. Pure ground glass nodular adenocarcinomas: Are preoperative positron emission tomography/computed tomography and brain magnetic resonance imaging useful or necessary? J Thorac Cardiovasc Surg 2015;150:514-20. [Crossref] [PubMed]
- Chun EJ, Lee HJ, Kang WJ, et al. Differentiation between malignancy and inflammation in pulmonary ground-glass nodules: The feasibility of integrated 18F-FDG PET/CT. Lung Cancer 2009;65:180-6. [Crossref] [PubMed]
- McDermott S, Kilcoyne A, Wang Y, et al. Comparison of the 18 F-FDG avidity at PET of benign and malignant pure ground-glass opacities: a paradox? Clin Radiol 2019;74:187-95. [Crossref] [PubMed]
- Sato M, Murayama T, Nakajima J. Concepts and techniques: How to determine and identify the appropriate target segment in anatomical pulmonary segmentectomy? J Thorac Dis 2019;11:972-86. [Crossref] [PubMed]
- El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol 2007;14:2400-5. [Crossref] [PubMed]
- Goldstein NS, Ferkowicz M, Kestin L, et al. Wedge resection margin distances and residual adenocarcinoma in lobectomy specimens. Am J Clin Pathol 2003;120:720-4. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Yoshida Y, Inoh S, Murakawa T, et al. Preoperative localization of small peripheral pulmonary nodules by percutaneous marking under computed tomography guidance. Interact CardioVasc Thorac Surg 2011;13:25-8. [Crossref] [PubMed]
- Sortini D, Feo C, Maravegias K, et al. Intrathoracoscopic localization techniques. Review of literature. Surg Endosc 2006;20:1341-7. [Crossref] [PubMed]
- Oikawa T, Nomoto Y, Kinoshita K. A case of left-sided hemiplegia due to cerebral air embolism caused by CT-guided marking for small peripheral lung cancer. Jpn J Chest Surg 2008;22:914-9. [Crossref]
- Kondo T, Tokunaga Y, Saito M, et al. Two cases of air embolism during percutaneous pulmonary marking under computed tomography guidance. Jpn J Chest Surg 2012;26:31-5. [Crossref]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomographyguided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Kuo SW, Tseng YF, Dai KY, et al. Electromagnetic Navigation Bronchoscopy Localization Versus Percutaneous CT-Guided Localization for Lung Resection via Video-Assisted Thoracoscopic Surgery: A Propensity-Matched Study. J Clin Med 2019;8:379. [Crossref] [PubMed]
- Yang SM, Yu KL, Lin KS, et al. Cumulative experience of preoperative real-time augmented fluoroscopy-guided endobronchial dye marking for small pulmonary nodules: An analysis of 30 initial patients. J Formos Med Assoc 2019;118:1232-8. [Crossref] [PubMed]
- Yang SM, Yu KL, Lin KH, et al. Real-time augmented fluoroscopy-guided lung marking for thoracoscopic resection of small pulmonary nodules. Surg Endosc 2020;34:477-84. [Crossref] [PubMed]
- Sato M, Kuwata T, Yamanashi K, et al. Safety and reproducibility of virtual-assisted lung mapping: a multicentre study in Japan. Eur J Cardiothorac Surg 2017;51:861-8. [Crossref] [PubMed]
- Sato M, Kobayashi M, Kojima F, et al. Effect of virtual-assisted lung mapping (VAL-MAP) in acquisition of surgical margins in sublobar lung resection. J Thorac Cardiovasc Surg 2018;156:1691-1701.e5. [Crossref] [PubMed]
- Sato M, Nagayama K, Kobayashi M, et al. Virtual-assisted lung mapping 2.0: preoperative bronchoscopic three-dimensional lung mapping. Ann Thorac Surg 2019;108:269-73. [Crossref] [PubMed]
- Ueda K, Uemura Y, Sato M. Protocol for the VAL-MAP 2.0 trial: a multicentre, single-arm, phase III trial to evaluate the effectiveness of virtual assisted lung mapping by bronchoscopic dye injection and microcoil implementation in patients with small pulmonary nodules in Japan. BMJ Open 2019;9:e028018 [Crossref] [PubMed]
- Varoli F, Vergani C, Caminiti R, et al. Management of solitary pulmonary nodule. Eur J Cardiothorac Surg 2008;33:461-5. [Crossref] [PubMed]
- Trejo Bittar HE, Incharoen P, Althouse AD, et al. Accuracy of the IASLC/ ATS/ERS histological subtyping of stage I lung adenocarcinoma on intraoperative frozen sections. Mod Pathol 2015;28:1058-63. [Crossref] [PubMed]
- Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤ 3 cm: accuracy and interobserver agreement. Histopathology 2015;66:922-38. [Crossref] [PubMed]
- Marchevsky AM, Changsri C, Gupta I, et al. Frozen section diagnoses of small pulmonary nodules: accuracy and clinical implications. Ann Thorac Surg 2004;78:1755-9. [Crossref] [PubMed]
- Ye T, Deng L, Wang S, et al. Lung Adenocarcinomas Manifesting as Radiological Part-Solid Nodules Define a Special Clinical Subtype. J Thorac Oncol 2019;14:617-27. [Crossref] [PubMed]
- Sano Y, Shigematsu H, Okazaki M, et al. Hoarseness after radical surgery with systematic lymph node dissection for primary lung cancer. Eur J Cardiothorac Surg 2019;55:280-5. [Crossref] [PubMed]
- Cho HJ, Kim DK, Lee GD, et al. Chylothorax complicating pulmonary resection for lung cancer: Effective management and pleurodesis. Ann Thorac Surg 2014;97:408-13. [Crossref] [PubMed]
- Milanese G, Sverzellati N, Pastorino U, et al. Adenocarcinoma in pure ground glass nodules: Histological evidence of invasion and open debate on optimal management. J Thorac Dis 2017;9:2862-7. [Crossref] [PubMed]
- Zha J, Xie D, Xie H, et al. Recognition of “aggressive” behavior in “indolent” ground glass opacity and mixed density lesions. J Thorac Dis 2016;8:1460-8. [Crossref] [PubMed]
- Song CY, Kimura D, Sakai T, et al. Novel approach for predicting occult lymph node metastasis in peripheral clinical stage I lung adenocarcinoma. J Thorac Dis 2019;11:1410-20. [Crossref] [PubMed]
- Suzuki S, Sakurai H, Yotsukura M, et al. Clinical features of ground glass opacity-dominant lung cancer exceeding 3.0 cm in the whole tumor size. Ann Thorac Surg 2018;105:1499-506. [Crossref] [PubMed]
- Moon Y, Sung SW, Namkoong M, et al. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis 2016;8:2617-25. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Consolidation/Tumor Ratio on Chest Computed Tomography as Predictor of Postoperative Nodal Upstaging in Clinical T1N0 Lung Cancer. World J Surg 2018;42:2872-8. [Crossref] [PubMed]
- Jiang W, Chen X, Xi J, et al. Selective mediastinal lymphadenectomy without intraoperative frozen section examinations for clinical stage I non-small-cell lung cancer: Retrospective study of 403 cases. World J Surg 2013;37:392-7. [Crossref] [PubMed]
- Han H, Zhao Y, Chen H. Selective versus systematic lymph node dissection (other than sampling) for clinical N2-negative non-small cell lung cancer: A meta-analysis of observational studies. J Thorac Dis 2018;10:3428-35. [Crossref] [PubMed]
- Deng HY, Qin CL, Li G, et al. Can lobe-specific lymph node dissection be an alternative to systematic lymph node dissection in treating early-stage non-small cell lung cancer: A comprehensive systematic review and meta-analysis? J Thorac Dis 2018;10:2857-65. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Franklin WA, et al. The IASLC lung cancer staging project: Summary of proposals for revisions of the classification of lung cancers with multiple pulmonary sites of involvement in the forthcoming eighth edition of the TNM classification. J Thorac Oncol 2016;11:639-50.
- Roberts PF, Straznicka M, Lara PN, et al. Resection of multifocal non-small cell lung cancer when the bronchioloalveolar subtype is involved. J Thorac Cardiovasc Surg 2003;126:1597-602. [Crossref] [PubMed]
- Shimada Y, Saji H, Otani K, et al. Survival of a surgical series of lung cancer patients with synchronous multiple ground-glass opacities, and the management of their residual lesions. Lung Cancer 2015;88:174-80. [Crossref] [PubMed]
- Hattori A, Matsunaga T, Takamochi K, et al. Radiological classification of multiple lung cancers and the prognostic impact based on the presence of a ground glass opacity component on thin-section computed tomography. Lung Cancer 2017;113:7-13. [Crossref] [PubMed]
- Li M, Wan Y, Zhang L, et al. Synchronous multiple lung cancers presenting as multifocal pure ground glass nodules: are whole-body positron emission tomography/computed tomography and brain enhanced magnetic resonance imaging necessary? Transl lung cancer Res 2019;8:649-57. [Crossref] [PubMed]
- NCCN. Clinical practice guidelines in oncology: Non- small cell lung cancer (Version 2.2019). 2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Eriguchi D, Shimada Y, Imai K, et al. Predictive accuracy of lepidic growth subtypes in early-stage adenocarcinoma of the lung by quantitative CT histogram and FDG-PET. Lung Cancer 2018;125:14-21. [Crossref] [PubMed]
- Fu L, Alam MS, Ren Y, et al. Utility of Maximum Standard Uptake Value as a Predictor for Differentiating the Invasiveness of T1 Stage Pulmonary Adenocarcinoma. Clin Lung Cancer 2018;19:221-9. [Crossref] [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Tumour standardized uptake value on positron emission tomography is a novel predictor of adenocarcinoma in situ for c-Stage IA lung cancer patients with a part-solid nodule on thin-section computed tomography scan. Interact Cardiovasc Thorac Surg 2014;18:329-34. [Crossref] [PubMed]
- Shiono S, Yanagawa N, Abiko M, et al. Detection of non-aggressive stage IA lung cancer using chest computed tomography and positron emission tomography/computed tomography. Interact Cardiovasc Thorac Surg 2014;19:637-43. [Crossref] [PubMed]
- Gao RW, Berry MF, Kunder CA, et al. Survival and risk factors for progression after resection of the dominant tumor in multifocal, lepidic-type pulmonary adenocarcinoma. J Thorac Cardiovasc Surg 2017;154:2092-2099.e2. [Crossref] [PubMed]
- Suzuki K. Whack-a-mole strategy for multifocal ground glass opacities of the lung. J Thorac Dis 2017;9:S201-7. [Crossref] [PubMed]
- Tane S, Nishio W, Nishioka Y, et al. Evaluation of the residual lung function after thoracoscopic segmentectomy compared with lobectomy. Ann Thorac Surg 2019;108:1543-50. [Crossref] [PubMed]
- Aridgides P, Bogart J. Stereotactic Body Radiation Therapy for Stage I Non-Small Cell Lung Cancer. Thorac Surg Clin 2016;26:261-9. [Crossref] [PubMed]
- Haasbeek CJ, Lagerwaard FL, Antonisse ME, et al. Stage I non-small cell lung cancer in patients aged ≥75 years: outcomes after stereotactic radiotherapy. Cancer 2010;116:406-14. [Crossref] [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Impact of introducing stereotactic lung radiotherapy for elderly patients with stage I non-small cell lung cancer: a population-based time-trend analysis. J Clin Oncol 2010;28:5153-9. [Crossref] [PubMed]
- Guckenberger M, Allgäuer M, Appold S, et al. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: a patterns-of-care and outcome analysis. J Thorac Oncol 2013;8:1050-8. [Crossref] [PubMed]
- Ricardi U, Frezza G, Filippi AR, et al. Stereotactic ablative radiotherapy for stage I histologically proven non-small cell lung cancer: an Italian multicenter observational study. Lung Cancer 2014;84:248-53. [Crossref] [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [Crossref] [PubMed]
- Ricardi U, Badellino S, Filippi AR. Stereotactic body radiotherapy for early stage lung cancer: History and updated role. Lung Cancer 2015;90:388-96. [Crossref] [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [Crossref] [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [Crossref] [PubMed]
- Mokhles S, Verstegen N, Maat A, et al. Comparison of clinical outcome of stage I non-small cell lung cancer treated surgically or with stereotactic radiotherapy: results from propensity score analysis Lung Cancer 2015;87:283-9. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Palma DA, Nguyen TK, Louie AV, et al. Measuring the Integration of Stereotactic Ablative Radiotherapy Plus Surgery for Early-Stage Non-Small Cell Lung Cancer: A Phase 2 Clinical Trial. JAMA Oncol 2019;5:681-8. [Crossref] [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Radiofrequency ablation of lung malignancies: where do we stand? Cardiovasc Intervent Radiol 2004;27:581-90. [Crossref] [PubMed]
- Sharma A, Abtin F, Shepard JA. Image-guided ablative therapies for lung cancer. Radiol Clin North Am 2012;50:975-99. [Crossref] [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [Crossref] [PubMed]
- Beland MD, Wasser EJ, Mayo-Smith WW, et al. Primary nonesmall cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology 2010;254:301-7. [Crossref] [PubMed]
- Huang L, Han Y, Zhao J, et al. Is radiofrequency thermal ablation a safe and effective procedure in the treatment of pulmonary malignancies? Eur J Cardiothorac Surg 2011;39:348-51. [Crossref] [PubMed]
- Jones GC, Kehrer JD, Kahn J, et al. Primary treatment options for high-risk/medically inoperable early stage NSCLC patients. Clinical Lung Cancer 2015;16:413-30. [Crossref] [PubMed]
- Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol 2009;38:135-43. [Crossref] [PubMed]
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 2011;261:643-51. [Crossref] [PubMed]
- Lu Q, Cao W, Huang L, et al. CT-guided percutaneous microwave ablation of pulmonary malignancies: Results in 69 cases. World J Surg Oncol 2012;10:80. [Crossref] [PubMed]
- Ricco A, Davis J, Rate W, et al. Lung metastases treated with stereotactic body radiotherapy: the RSSearch® patient Registry's experience. Radiat Oncol 2017;12:35. [Crossref] [PubMed]


