
Large nest micropapillary pattern of lung adenocarcinoma has poorer prognosis than typical floret pattern: analysis of 1,062 resected tumors
Introduction
Lung adenocarcinoma (L-ADC) is the most common histological subtype of primary lung cancer. It exhibits molecular, clinical, radiological, surgical, and pathological heterogeneity (1). The micropapillary pattern (MP-p) of L-ADC was initially reported in 2002 (2) and has been described to have a poor prognostic pattern (3-9). MP adenocarcinoma was proposed as a new histological subtype of L-ADC by the International Association for the Study of Lung Cancer, American Thoracic Society, and the European Respiratory Society in 2011 (10); additionally, the World Health Organization (WHO) renewed its classification in 2015 (1). The core feature of MP-p is “small papillary tufts with no fibrovascular cores appearing detached from alveolar wall”. However, the MP-p criteria fail to define a “large tumor cell nest which does not form a pseudo papillary structure”. This flaw in the MP-p definition may cause considerable discrepancies in MP-p diagnosis among different observers (11-13).
In the present study, we divided floating tumor clusters in the air space into two types based on their size and investigated the effect of these types on clinical outcomes by histologically reviewing 1,062 resected L-ADCs.
Methods
Cohort
A retrospective analysis was carried out on patients with L-ADC who underwent complete resection with curative intent at Kyoto University Hospital between 2001 and 2015. Patients who had multiple primary lung cancers, were treated with chemotherapy or radiotherapy before surgery, underwent incomplete resection, or had incomplete follow-up information in the clinical data retrieved from the Thoracic Surgical Database were excluded from the study. The analysis ultimately included 1,062 L-ADCs. The study protocol was approved by the Kyoto University Hospital ethics committee (R1158-1).
Histological evaluation
All resected specimens were fixed in formalin, sectioned, and stained with hematoxylin and eosin according to standard procedures. Small tumors were histologically sampled as one sample. Elastic staining was performed to detect pleural or vessel invasion. All specimens were reviewed by two pathologists (KK and AY) who were blinded to patient information, and all histological parameters were established by consensus after discussion. The average number of tumor specimens reviewed for each case was 3.3 (range, 1–20). According to the 2015 WHO classification (1), each tumor was subjected to comprehensive histological subtyping, and the percentage of each histological component was recorded in 5% increments.
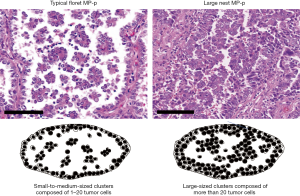
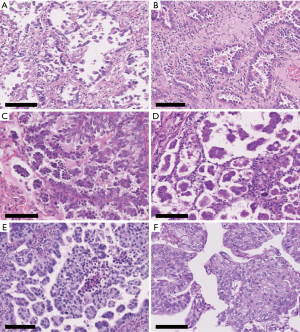
Two MP-p patterns were defined according to cluster size in the air space as follows: small to medium-sized cluster (composed of 1–20 tumor cells), typical floret MP-p (10,14), and large-sized cluster (composed of more than 20 tumor cells), large nest MP-p (Figure 1). Before the review, we assessed 100 cases and observed air space tumor clusters of various sizes in individual cases. We found single cells or small clusters (composed of 2–3 tumor cells) showing MP-p-like feature (Figure 2A,B). Because this feature may be an artifact appearing as tangential cells or small clusters of lepidic or papillary patterns, we classified this structure as absent of Mp-p in this study when a typical floret Mp-p was not identified. Next, we recorded typical floret MP-p when a small-to-medium-sized cluster was observed without a large-sized cluster, and large nest MP-p when a large-sized cluster was observed in the presence or absence of small to medium-sized cluster (Figure 1). We also included the stromal MP-p as an additional intra-alveolar floret MP-p (Figure 2C,D). Moreover, although the feature of large nest MP-p may be considered as an artifact showing pseudostratified tumor cells of acinar or solid patterns, we classified this structure as a new pattern in this study (Figure 2E,F). Based on the criteria, we assessed the whole specimens and recorded the percentage of MP-p in 5% increments. We then investigated the association between MP-p type and the following clinicopathological factors: sex, age, smoking status, tumor grade, histological subtype, lymphatic/vascular/pleural invasion, spread through air spaces (STAS) according to the 2015 WHO classification (1), and tumor-node-metastasis (TNM) staging according to the 8th TNM classification (15).
Detection of genetic alterations in various oncogenes
The association between tumors with MP-p and mutations in the epidermal growth factor receptor (EGFR), Kirsten rat sarcoma viral oncogene homolog (KRAS), human epidermal growth factor receptor 2 (HER2), B-raf proto-oncogene serine/threonine protein kinase (BRAF), anaplastic lymphoma kinase (ALK), and ROS proto-oncogene 1 (ROS1) was evaluated according to our previous studies (16-21). Briefly, EGFR mutations were evaluated by polymerase chain reaction single-strand conformation polymorphism (PCR-SSCP) before 2009 (18) and the PNA-LNA PCR clamp method after 2010. HER2 and BRAF mutations were also examined by PCR-SSCP (17). KRAS mutation was investigated using a modified mutagenic PCR restriction fragment length polymorphism technique (18). ALK fusion was detected by reverse transcription PCR and fluorescence in situ hybridization (FISH) (16, 20). ROS1 fusion was detected by FISH using ROS1 Dual Color Break Apart Probe (Vysis LSI/Abbott Laboratories, Chicago, IL, USA) according to the manufacturer’s instructions (19). Ret proto-oncogene (RET) fusion was also detected by FISH using the Kreatech RET (10q11) Break FISH probe (Leica Biosystems, Wetzlar, Germany) and RET Split Dual Color FISH probe (GSP Lab., Tokyo, Japan) according to the manufacturer’s instructions.
Statistics
The χ2 and Fisher’s exact tests were applied to analyze categorical data. Survival rates were calculated by the Kaplan-Meier method, and differences were analyzed with the log-rank test. Multivariate analysis was performed using Cox’s proportional hazards model. Multivariate models were generated to include factors that were significant in univariate analysis. All statistical tests were two-sided at a 5% level of significance. Data analysis was performed using JMP v.13 statistical software package (SAS Institute, Cary, NC, USA). Summary graphs were generated using JMP v.13 statistical software package and Microsoft PowerPoint 2016 (Microsoft Corporation, Redmond, WA, USA).
Results
Clinicopathological characteristics
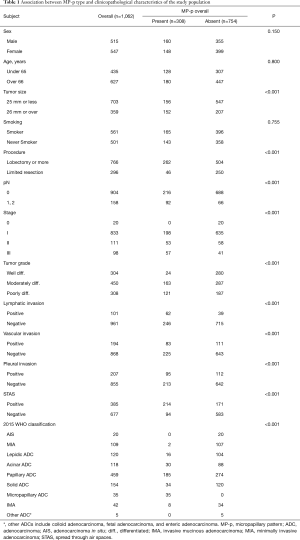
The clinicopathological characteristics of the study population are shown in Table 1. There were 515 (48.5%) male and 547 (51.5%) female patients; the mean age at diagnosis was 66.2±9.8 years (range, 23–88 years); mean tumor size was 23.9±14 mm (range, 3–120 mm); and 561 (52.8%) patients were smokers (221 current smokers, 340 ex-smokers; smoking index =45.7). Most patients underwent lobectomy of one or more lobes (n=766, 72.1%), and the others (n=296, 27.8%) underwent limited resection (segmentectomy or wedge resection). A total of 186 (17.3%) patients died during follow-up, and 241 (22.4%) relapsed. The mean follow-up time at the end-point of analysis was 61.5±35.7 months. The number of patients at each pathologic stage was as follows: stage 0, 20 (1.9%) patients; I, 832 (78.3%) patients; II, 111 (10.5%) patients; and III, 98 (9.2%) patients.
Full table
Correlation between MP-p type and clinicopathological characteristics
Of the 1,062 cases, MP-p was present in 308 tumors (29.0%), predominantly in those with a larger tumor size (P<0.001), exhibiting lymph node metastasis (P<0.001), higher pathological stage (P<0.001), lymphatic invasion (P<0.001), vascular invasion (P<0.001), pleural invasion (P<0.001), and STAS (P<0.001). There was no association between the presence of MP-p and age, sex, or smoking status (Table 1).
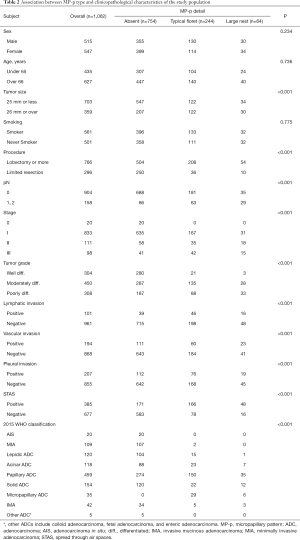
Table 2 and Figure 3 show the correlations between clinicopathological factors and detailed MP-p types. Typical floret MP-p was observed in 244 tumors (22.9% of all tumors), whereas large nest MP-p was observed in 64 tumors (6.0% of all tumors). The range of percentage of typical floret MP-p and large nest Mp-p were 5–95% with a mean of 18.3 [standard deviation (SD) 17.9] and 5–80% with a mean of 19.1 (SD 14.9), respectively. We found that lymph node metastasis was most frequently associated with large nest MP-p (45.3%), followed by typical floret MP-p (25.8%) and absent of MP-p (8.7%; Figure 3A). In subclass analysis with tumors with typical floret MP-p and large nest MP-p, lymph node metastasis was significantly observed in tumors with large nest MP-p in contrast to tumors with typical floret MP-p (P=0.003). Lymphatic, vascular, and pleural invasion were more frequent in tumors with typical floret MP-p and large nest MP-p compared to those without MP-p, whereas no significant difference was observed between tumors with typical floret MP-p and those with large nest MP-p (Figure 3B,C,D). STAS was most frequently detected in tumors with large nest MP-p (75.0%), followed by tumors with typical floret MP-p (68.0%) and tumors without MP-p (22.6%) (P<0.001; Figure 3E). Large nest MP-p was frequently present in advanced stage tumors, whereas MP-p were rarely observed in stage I tumors (P<0.001; Figure 3F).
Full table
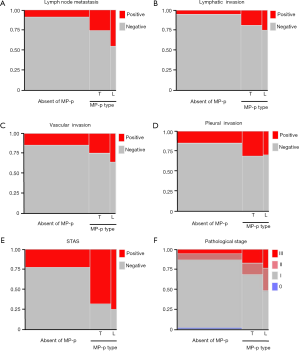
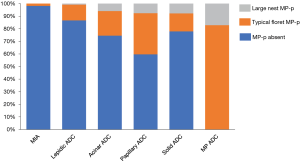
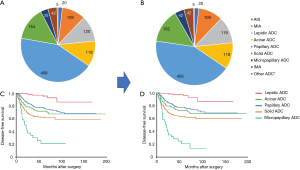
Figure 4 shows the incidence of MP-p type by adenocarcinoma subtype. MP-p was frequently observed in tumors of papillary adenocarcinoma (40.3%) except for micropapillary adenocarcinoma, followed by acinar adenocarcinoma (25.4%) and solid adenocarcinoma (22.7%). We reclassified the tumors by including large nest MP-p in the classical MP-p. The number of cases of micropapillary ADC was increased by 7 (tumor incidence: 3.95%) compared to the initial number of cases (tumor incidence: 3.29%). The number of cases of lepidic ADC, papillary ADC, and solid ADC was decreased by 1, 4, and 2, respectively (Figure 5A,B). The 3- and 5-year disease-free survival rates of patients with reclassified MP ADC were 31.6% and 20.8%, respectively, which did not differ from those of patients with original MP ADC (33.7% and 20.6%, respectively) (Figure 5C,D).
Association between presence of MP-p and alterations in oncogenes
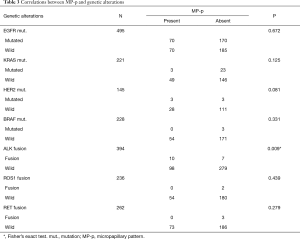
In the 1,062 L-ADC specimens, we observed mutations in EGFR (240/495, 48.5%), KRAS (26/221, 11.8%), HER2 (6/145, 4.1%), and BRAF (3/228, 1.3%) as well as fusions in ALK (17/394, 4.3%), ROS1 (2/236, 0.8%), and RET (3/262, 1.1%). We found a correlation between MP-p-positive tumors and ALK fusion (P=0.009, Fisher’s exact test), but not between MP-p-positive tumors and EGFR (P=0.672), KRAS (P=0.125), HER2 (P=0.081), or BRAF (P=0.331) mutation and ROS1 (P=0.439) or RET (P=0.279) fusion (Table 3).
Full table
MP-p type and patient outcome
We investigated the association between survival and MP-p status in cases of invasive adenocarcinoma except for invasive mucinous adenocarcinoma and other variants (n=886). We found that patients with MP-p-positive tumors had worse prognosis than those with MP-p-negative tumors both in terms of disease-free survival (DFS) and overall survival (OS) (5-year DFS rate, 56.8%, P<0.001; 5-year OS rate, 76.0%; P<0.001; Figure 6A,B). Patients with large nest MP-p tumors had the worst prognosis (5-year DFS rate, 39.7%), followed by those with typical floret MP-p tumors (5-year DFS rate, 60.2%), whereas those without MP-p had better prognosis (5-year DFS rate, 82.6%) (Figure 6C). Patients with large nest MP-p tumors had the worst prognosis (5-year OS rate, 66.8%) followed by those with typical floret MP-p tumors (5-year OS rate, 78.3%), whereas those without MP-p had better prognosis (5-year OS rate, 87.7%) (Figure 6D).
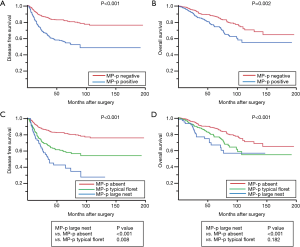
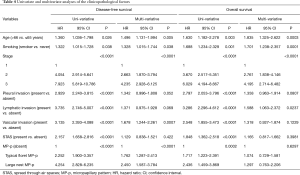
Table 4 shows the results of the uni- and multivariate analyses of the clinicopathological factors examined in this study. Based on the results of univariate analysis, we performed multivariate analyses using the Cox proportional hazards model and found that age, smoking status, stage, vascular invasion, and MP-p were independently associated with recurrence risk [typical floret MP-p vs. MP-p absent, hazard ratio (HR): 1.762, 95% confidence interval (CI), 1.287–2.413; large nest MP-p vs. MP-p absent, HR: 2.450, 95% CI, 1.587–3.784, P<0.0001]. Moreover, age, smoking status, stage, and lymphatic invasion were independent prognostic factors of worse OS; however, MP-p was not a significant independent prognostic factor for OS (P=0.629), although it was significant in univariate analysis (P=0.0002).
Full table
Discussion
In this study, we demonstrated that MP-p is present in 29.0% of L-ADCs, with typical floret MP-p as the most common. We found that MP-p is an independent predictor of a worse clinical outcome for recurrent disease in patients with resected L-ADC and is associated with aggressive tumor characteristics such as large tumor size, advanced pathological stage, lymph node metastasis, pleural invasion, lymphovascular invasion, and STAS. The frequency and prognosis of reclassified micropapillary ADC slightly differed from those of the original micropapillary ADC. Further, our results showed that patients with large nest MP-p experienced recurrence more frequently than those with typical floret MP-p and without MP-p. These findings highlight the prognostic value of classifying MP-p type according to cluster size of the air space.
Although the current WHO classification briefly references the micropapillary histological subtype (1), considerable inter-observer variability is common in the identification of MP-p (11-13). Thunnissen et al. reported that the concordance rate of the micropapillary subtype was lower (62%) than those of the other subtypes (92–100%) (13); the authors noted that only 12% of participant pathologists in the study identified MP-p as a single pattern. This may be because of flaws in the MP-p definition, which prompted the current study.
In routine clinical practice, single cells and small clusters (composed of 2–3 tumor cells) floating within tumor glands (Figure 2A,B), resembling a MP-p, are often observed and confuse pathologists. This pattern does not represent a typical MP-p, as it is not mentioned in the WHO classification. In Thunnissen’s report, we found some interesting images showing tiny tumor cell clusters mixed with the other growth patterns (Figure 3g and 3j in ref. 12) (12). Warth et al. also reported that this pattern was particularly challenging for distinguishing between papillary structures and MP growth (Figure 2c in ref. 11) (11). However, this pattern type has never been studied to determine its clinical significance. In the current study, we explored the prognostic significance and found DFS and OS curves between tumors with single cells and small clusters and without MP-p were not separated (data not shown). This may be because the feature is an artifact showing tangential cells or small peel-off clusters of lepidic or papillary patterns. Thus, single cells and small clusters should not be considered as a part of MP-p.
In contrast, large-sized clusters comprising over 20 tumor cells within the tumor nest or air space are often observed in poorly differentiated L-ADC; however, the clinical significance of this pattern has not been determined. These cases may represent a spectrum of MP-p because they are often admixed in the same tumor and appear to arise from tumor cells that have detached from the wall of tumor glands or papillae. In this study, we classified this pattern as large nest MP-p and evaluated its clinical significance. Tumors with large nest MP-p were rare (6%) but strongly influenced the risk of recurrence and death. Further, patients with large nest MP-p tumors had a worse prognosis than those with typical floret MP-p tumor. These findings indicate that large nest MP-p should be categorized as MP-p and separately from typical floret MP-p.
STAS has recently been recognized as an invasive pattern of lung cancer. It is a prognostic factor in patients who have undergone limited resection (22,23). In 2015, in the WHO classification of lung tumor fascicles, STAS was defined as “micropapillary clusters, solid nests, or single cells extending beyond the edge of the tumor into air spaces” (1). The classification is conceptually similar to our extended MP-p concepts, as it also focuses on floating tumor cell clusters of variable size, although STAS is proposed to occur outside the tumor mass. In our study, STAS was most frequently observed in tumors with large nest MP-p, followed by those with typical floret MP-p, and was rarely observed in tumors without MP-p. Thus, larger clusters presumably spread beyond the edge of the tumor mass. Interestingly, both STAS and MP-p were risk factors for recurrence according to univariate analysis; however, STAS was eliminated as an independent risk factor in multivariate analysis. This indicates that although they are closely correlated, MP-p is a stronger risk factor for recurrence than STAS and may have prognostic value. To clarify this hypothesis, additional studies examining the association between the size of floating cell clusters inside (MP-p) and those outside (STAS) of the mass are needed.
The filigree pattern is a newly proposed addition to the morphological spectrum of micropapillary ADCs with poor prognosis (24). This pattern is defined as tumor cells growing in delicate lace-like narrow stacks of cells (at least three stacked nuclei) without fibrovascular cores, with visible attachments to alveolar walls. No significant differences in prognosis were observed between the filigree (reclassified from papillary, acinar, and solid predominant adenocarcinoma) and classical type MP-predominant groups. In the current study, we did not evaluate this distinct subtype of MP-p. Because the prognostic significance of the filigree pattern in adenocarcinoma has not been extensively studied, additional studies are needed to validate these definitions along with large nest MP-p.
There were some limitations to this study. First, it may be challenging to accurately distinguish large nest MP-p from typical floret MP-p; however, there were a few disagreements among the observers regarding the identification and assessment of MP-p. This may be because we had a "consensus" track with 100 cases; future studies are required to validate these findings. Second, some researchers recently reported that STAS, as well as detached tumor cells inside the tumor area, were identified as a sampling artifact caused by gross pathology preparation (25). Although we did not examine this point in detail, tumors with discohesive or easy-detaching character should be recorded even if they are generated from artifacts. Further multiple institute analyses are required. Third, when samples are not sufficiently fixed in formalin solution, tumor cells may appear to be floating in the stroma (a so-called fixation artifact). In this study, we did not consider that such an artifact constituted a feature of MP-p. Moreover, previous studies of MP adenocarcinoma of the lung have not evaluated this possibility, which should be further examined. Fourth, in mucinous type tumors, particularly invasive mucinous adenocarcinoma, floating tumor nests are often observed in the extracellular mucin. In this study, we did not consider such floating nests as a feature of MP-p because the frequency of invasive mucinous adenocarcinoma was low (3.8% in the current study); however, further examination of the significance of floating nests in the mucinous tumor are needed.
Conclusions
We identified only 7 more micropapillary ADC cases when we reclassified ADCs in addition to large nest MP-p; however, a lack of significant prognostic differences between classical and reclassified micropapillary ADC was observed. These findings support the expansion of morphological criteria for micropapillary ADC to include large nest MP-p. This expansion may help to achieve a good concordance in the recognition of the MP-p. Further, the present study demonstrated that MP-p is an independent factor for predicting the recurrence and poor prognosis that may follow resection of L-ADC. We also showed that tumors with large nest MP-p were related to the highest recurrence rate compared to tumors without MP-p and those with typical floret MP-p. We consider that MP-p should be separately recorded according to their size regardless of the tumor subtype.
Acknowledgments
Funding: This work was supported in part by a Japan Society for the Promotion of Science KAKENHI grant (17K08740).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-19-731). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Kyoto University Hospital (approval number: R1158-1). This article is a retrospective analysis, so informed consent is waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Geneva: IARC Press, 2015.
- Amin MB, Tamboli P, Merchant SH, et al. Micropapillary component in lung adenocarcinoma: a distinctive histologic feature with possible prognostic significance. Am J Surg Pathol 2002;26:358-64. [Crossref] [PubMed]
- Tsutsumida H, Nomoto M, Goto M, et al. A micropapillary pattern is predictive of a poor prognosis in lung adenocarcinoma, and reduced surfactant apoprotein A expression in the micropapillary pattern is an excellent indicator of a poor prognosis. Mod Pathol 2007;20:638-47. [Crossref] [PubMed]
- Sumiyoshi S, Yoshizawa A, Sonobe M, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer 2013;81:53-9. [Crossref] [PubMed]
- Sánchez-Mora N, Presmanes MC, Monroy V, et al. Micropapillary lung adenocarcinoma: a distinctive histologic subtype with prognostic significance. Case series. Hum Pathol 2008;39:324-30. [Crossref] [PubMed]
- Miyoshi T, Satoh Y, Okumura S, et al. Early-stage lung adenocarcinomas with a micropapillary pattern, a distinct pathologic marker for a significantly poor prognosis. Am J Surg Pathol 2003;27:101-9. [Crossref] [PubMed]
- Makimoto Y, Nabeshima K, Iwasaki H, et al. Micropapillary pattern: a distinct pathological marker to subclassify tumours with a significantly poor prognosis within small peripheral lung adenocarcinoma (</=20 mm) with mixed bronchioloalveolar and invasive subtypes (Noguchi's type C tumours). Histopathology 2005;46:677-84. [Crossref] [PubMed]
- Kawakami T, Nabeshima K, Makimoto Y, et al. Micropapillary pattern and grade of stromal invasion in pT1 adenocarcinoma of the lung: usefulness as prognostic factors. Mod Pathol 2007;20:514-21. [Crossref] [PubMed]
- Kamiya K, Hayashi Y, Douguchi J, et al. Histopathological features and prognostic significance of the micropapillary pattern in lung adenocarcinoma. Mod Pathol 2008;21:992-1001. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Warth A, Cortis J, Fink L, et al. Training increases concordance in classifying pulmonary adenocarcinomas according to the novel IASLC/ATS/ERS classification. Virchows Arch 2012;461:185-93. [Crossref] [PubMed]
- Urer HN, Ahiskali R, Arda N, et al. Interobserver agreement among histological patterns and diagnosis in lung adenocarcinomas. Turk Patoloji Derg 2014;30:105-10. [PubMed]
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [Crossref] [PubMed]
- Hoshi R, Tsuzuku M, Horai T, et al. Micropapillary clusters in early-stage lung adenocarcinomas: a distinct cytologic sign of significantly poor prognosis. Cancer 2004;102:81-6. [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant tumours Eighth Edition. Oxford: Wiley-Blackwell, 2017.
- Takahashi T, Sonobe M, Kobayashi M, et al. Clinicopathologic features of non-small-cell lung cancer with EML4-ALK fusion gene. Ann Surg Oncol 2010;17:889-97. [Crossref] [PubMed]
- Sonobe M, Manabe T, Wada H, et al. Lung adenocarcinoma harboring mutations in the ERBB2 kinase domain. J Mol Diagn 2006;8:351-6. [Crossref] [PubMed]
- Sonobe M, Kobayashi M, Ishikawa M, et al. Impact of KRAS and EGFR gene mutations on recurrence and survival in patients with surgically resected lung adenocarcinomas. Ann Surg Oncol 2012;19 Suppl 3:S347-54. [Crossref] [PubMed]
- Rokutan-Kurata M, Yoshizawa A, Sumiyoshi S, et al. Lung Adenocarcinoma With MUC4 Expression Is Associated With Smoking Status, HER2 Protein Expression, and Poor Prognosis: Clinicopathologic Analysis of 338 Cases. Clin Lung Cancer 2017;18:e273-81. [Crossref] [PubMed]
- Nakajima N, Yoshizawa A, Kondo K, et al. Evaluating the effectiveness of RNA in-situ hybridization for detecting lung adenocarcinoma with anaplastic lymphoma kinase rearrangement. Histopathology 2017;71:143-9. [Crossref] [PubMed]
- Kobayashi M, Sonobe M, Takahashi T, et al. Clinical significance of BRAF gene mutations in patients with non-small cell lung cancer. Anticancer Res 2011;31:4619-23. [PubMed]
- Warth A, Muley T, Kossakowski CA, et al. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. Am J Surg Pathol 2015;39:793-801. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katsuki N, et al. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Emoto K, Aly RG, Montecalvo J, et al. Clinical Significance of Morphological Patterns of Micropapillary Lung Adenocarcinoma: Classical and Filigree Patterns. Mod Pathol 2018;31:731.
- Blaauwgeers H, Russell PA, Jones KD, et al. Pulmonary loose tumor tissue fragments and spread through air spaces (STAS): Invasive pattern or artifact? A critical review. Lung Cancer 2018;123:107-11. [Crossref] [PubMed]


