
Bioengineered carina reconstruction using In-Vivo Bioreactor technique in human: proof of concept study
Introduction
Long-segment airway defect reconstruction, especially when carina is invaded, remains a challenge in a clinical setting. Previous attempts at bioengineered carina reconstruction failed within 90 days due to delayed revascularization and recurrent infection (1). Tissue engineering (TE) and regenerative medicine may provide a promising solution (2). Traditional TE techniques have two separate stages that include in vitro three-dimensional (3D) cell-scaffold culture and in vivo TE substitute regeneration. Since it is extremely difficult to create a capillary network in vitro, implanted TE substitutes can only obtain nutrition via tissue fluid infiltration and vessel in-growth from surrounding recipient tissue (3). Unfortunately, the revascularization process normally takes several months during which the majority of pre-seeded cells located in the central part of the TE substitute die from ischemia (4).
This fact brought into question the necessity of cell pre-seeding and gave birth to the in situ TE theory, which neglected cells and implanted only the shaped biodegradable scaffold together with growth factor(s) to induce in situ stem cell growth and differentiation. This modification was not very successful and demonstrated the critical function of cells in the regenerative process. Considering the fact that we age because of a decreased capacity for stem cell proliferation, it should not be surprising that we need cells for truly functional reconstruction. Delayed revascularization kills most cells within implanted constructs and prevents the clinical application of large TE prostheses. So far, successful clinical application of TE products has only been accomplished in cartilage tissue (which is avascular) and skin (which is placed on a well-vascularized-wound surface).
To solve this problem, an In-Vivo Bioreactor design described as implanted TE substitutes perfused with intra-scaffold medium flow created by two portable peristaltic pumps was introduced for in situ tissue regeneration (5). During pilot in vitro studies and animal experiments, three main advantages of this design were demonstrated. First, continuous medium perfusion maintained the pre-seeded cell survival. Second, the In-Vivo Bioreactor functioned as a cell re-seeding system through which cells could be repeatedly seeded into the implanted TE airway substitute. Third, antibiotics could be added into the perfusate to prevent infection (6,7).
Relying on prior experience with the In-Vivo Bioreactor bioengineered human left main bronchus reconstruction, a decision was made to attempt a repair of long-segment airway and carina defect (8). The Shanghai Chest Hospital Ethics Board approve a prove-of-concept clinical trial with maximum three cases.
Methods
Study design
The study was sponsored by the High-tech Three-year Action Project of Shanghai Hospital Development Center, project (No. 16CR3066B). The protocol was written by the principal investigator (Prof. QT). As a proof of concept study, the investigator initially planned to enroll three patients into this study for the funding application. So according to the project protocol, Shanghai Chest Hospital Ethics Board give permission for only three patients. The study was approved by institutional ethics board of No. KS1744 in November 2017 and informed consent was taken from all the patients. The protocol focused on bioengineered carina reconstruction.
Patients
Eligible patients underwent a standard preoperative evaluation and bronchoscopy tests. A multidisciplinary team approved the inclusion and exclusion criteria. According to the project plan, we have enrolled three patients into this study from 2017 to 2020. Patients were included in the study if they had (I) extensive airway tumor required trachea and carina resection longer than 6 cm that was not able to perform direct end-to-end anastomosis; (II) had proximal lung tumors requiring a surgical resection (pneumonectomy, carinal resection, or sleeve lobectomy) that had compromised preoperative lung function tests. Patients were excluded from the study if they (I) had airway tumor resected less than 6 cm and could be reconstructed through direct end-to-end anastomosis; (II) had a lung tumor requiring a standard lobectomy or sleeve lobectomy; (III) late-stage tumor with distant metastasis.
Finally, the ethical board approved pilot clinical trial with maximum three cases who were enrolled from December 2017 till July 2018. The patient characteristics, type of operation, and indications for inclusion summarize in Table 1. All the cases required carina reconstruction.
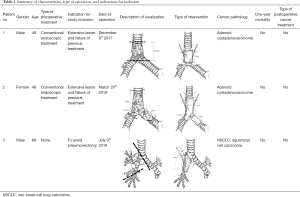
Full table
Treatment
After enrollment in the study, a custom-made nitinol stent (Beijing Puyishengji Technology Co., Ltd., Beijing, China) was manufactured based on preoperative computed tomography CT trachea 3D image. Radical resection of the airway lesion was performed using standard surgical techniques. The airway defect was reconstructed by bioengineered substitute with In-Vivo Bioreactor technique (Figure 1). Simply put, the custom-made stent was wrapped in two layers of the acellularized dermis matrix (ADM) (Unitrump Biomedical Technology Co., Ltd., Qidong, China) between which two Port-a-Cath (Smiths Medical, Minneapolis, MN, USA) catheters were inserted. The ends of the two Port-a-Cath catheters from the substitute were pulled out of the thorax and connected to the two ports embedded subcutaneously in chest wall. Immediately after the operation, two ambulatory infusion peristaltic pumps (ACE Medical Co., Ltd., Seoul, Korea) were connected to the subcutaneous ports using needles and transfusion tubes. One pump continuously delivered Ringer’s solution with 100 µg/mL gentamicin into the bioengineered airway substitute, while the other pump removed the waste. The speed of both pumps was adjusted to 10 mL/h.
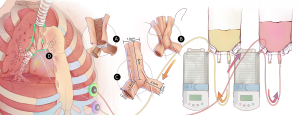
Procedures
The case 1 and 2 trachea adenoid cystic carcinoma (ACC) patients were placed in a supine position first. The omental flap was mobilized based on the right gastroepiploic vascular pedicle via the upper midline abdominal incision. The omental flap was then transferred into the right thorax through a substernal tunnel. After closing the abdominal incision, the patient was placed in a left posterolateral position. During an uniportal video-assisted thoracic surgery, a U-shaped incision was made around the left inferior pulmonary vein after dividing the left inferior pulmonary ligament. The patient was then placed in a right posterolateral position and a thoracotomy was performed via the bed of the resected fifth rib. The airway tumor was isolated and resected with negative margins. Once the trachea tumor was resected, we ventilated the left lung by catheter connected between ventilator and left main bronchus. When anastomosis completed, we ventilated through trachea to maintain ventilation. The airway defect was repaired with tracheal substitute with In-Vivo Bioreactor design. The whole tracheal substitute, including anastomoses, was buttressed with omentum.
Case 3 lung cancer patient was placed in a right posterolateral position and a thoracotomy was performed via the bed of the resected fifth rib. We performed right upper and middle bi-lobectomy and resection of right main bronchus and wedge resection of carina and lower part trachea. Once the bi-lobectomy and right main bronchus were resected, we ventilated the left lung by catheter connected between ventilator and left main bronchus. When anastomosis completed, we ventilated through trachea to maintain ventilation. The airway defect was repaired with tracheal substitute with In-Vivo Bioreactor design. The whole tracheal substitute, including anastomoses, was buttressed using intercostal muscle flap.
Follow-up and assessment of outcomes
Patients were followed up for 1 year. The outcomes of mortality and morbidity were assessed, including complications related to the bioengineered substitute and In-Vivo Bioreactor design. Bronchoscopy were performed every week for the first month and every 3 months for 1 year. CT scan were performed every 3 months.
The primary outcome was 1-year mortality and the secondary outcome was 1-year morbidity. After 1 year, the patients were follow-up for long-term mortality and morbidity outcomes. For patients, the last flow-up visit occurred on January, 2020.
Results
Primary outcome
The 1-year mortality was 0% and all three patients discharged from hospital back to normal life.
Secondary outcomes
The mean length of hospitalization was 60 days and range from 9 to 88 days. The mean In-Vivo Bioreactor perfusion day was 123 days and range from 72 to 196 days. Major 1-year morbidity events occurred in two ACC patients included gastric retention and pneumonia. To avoid the perfusate leakage into the lung and to prevent bacteria colonization in the inner ADM surface, Y-shaped membrane stents were placed in the two ACC patients on postoperative day 36 and 80 respectively (Figure 2).
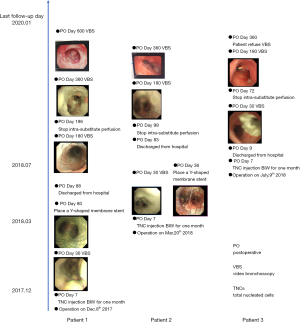
Long-term follow-up
Long-term follow-up did not identify any major complications related to bioengineered substitute and In-Vivo Bioreactor design. Granulomata located at the mainstem bronchi ends of the membrane stent was ablated using laser therapy every 3 months for the two ACC patients. Patients are uneventful and all are able to engage in usual activities. The bronchoscopy of case 1 patient postoperative 17 months is followed as Video 1. The bronchoscopy of case 2 patient postoperative 20 months is followed as Video 2.
The bronchoscopy follow-up shew nicely the gradually biodegradation process of ADM together with the regeneration process of granulation tissue. The last bronchoscopy follow-up of all the three patients found no ADM remain the substitutes are all replaced by the recipient’s granulation tissue.
Discussion
Delayed revascularization and lethal recurrent infection are major challenges for a successful long-segment airway reconstruction. Current trachea replacement methods can be categorized into four categories: allotransplantation, autologous tissue reconstruction, bioprosthetic reconstruction, and TE reconstruction (9). Each method has its own advantages and disadvantages and none are able to solve the two problems perfectly.
Allotransplantation requires long-term immunosuppression, making it unsuitable for malignant tumor patients. Delaere et al. demonstrated that indirect revascularization of donor trachea required around 4 months during which the donor trachea posterior membranous sheath underwent avascular necrosis (10). At the time of the orthotopic tracheal allotransplantation, recipient tissue replaced almost all of the donor trachea tissue leaving only a cartilaginous framework. Whether the allogeneic cartilage tissue will also be absorbed requires a long-term follow-up investigation. These results emphasized the importance of a well-vascularized buttress tissue for trachea allograft revascularization and demonstrated that the donor trachea will eventually be replaced by regenerated recipient tissue.
Fabre et al. reported on an 8-year study of the autologous tissue tracheal replacement method (11). Although the results are promising, two carina reconstruction patients died from severe pneumonia within 90 days. Therefore, this technique was not recommended for cases when the need for resection and reconstruction extends beyond the trachea itself. Repeated life-threatening episodes of pneumonia might occur due to an ischemic period causing epithelial tissue necrosis in an autologous tissue substitute during preparation. After implantation, the necrotic tissue is susceptible to bacterial colonization leading to lethal recurrent pneumonia. This study implied that direct vessel anastomosis revascularization method might not be efficient enough to maintain epithelial tissue viability.
Stented aortic matrix as reported by Martinod et al. is the most popular choice in the field of bioprosthetic reconstruction (12). Without direct vessel anastomosis, the bioprosthetic revascularization process depends on surrounding recipient tissue vessel in-growth. As already demonstrated during the allotransplantation trachea replacement method, revascularization normally takes several months, eventually leaving the airway substitute susceptible to infection. Therefore, the authors announced that this technique should be approached with extreme caution when used for carina reconstruction.
Similarly, traditional TE methods provide limited advantage in solving revascularization and anti-infection problems (13). Due to delayed revascularization, the majority of pre-seeded cells die from ischemia right after TE substitute implantation and consequently poison the local regeneration niche. It is also problematic that better biocompatible scaffolds facilitate both normal cell and bacteria ingrowth, making TE substitutes susceptible to infection.
In this context, this study utilized an “in vivo bioreactor” design integrating advantages of the four bioengineered airway reconstruction methods. The substitute was enveloped with omentum or intercostal muscle flap that proved to be superior revascularization buttress tissue types for the trachea allotransplantation method. In autologous tissue airway reconstruction methods, metal strips are often inserted into autologous tissue tracheal substitutes to provide mechanical strength. Similarly, instead of the cartilage tissue might be absorbed in the long-term, a nitinol stent was placed, inside the ADMs to avoid bioengineered airway collapse. Following the stented aortic matrix airway reconstruction method, a temporary membrane stent was inserted to prevent biodegraded ADM fragments depositing caudally into the lung and causing lethal pneumonia. Utilizing the TE principles, the importance of seeded cells in the regeneration process of the airway substitute was emphasized. Different from the traditional TE method, the in vitro 3D cell-scaffold culture was integrated with the in vivo substitute regeneration processes. The patients were treated as a bioreactor for her own airway substitute regeneration.
In a previous study, it was demonstrated that intra-substitute perfusion supported the survival of seeded cells that were able to secrete various growth factors accelerating the revascularization process (8). Bronchoscopy follow-up showed well-vascularized tissue formation 3 months after implantation, which was earlier than reported for the allotransplantation method. Based on the experience of chest lavage for empyema treatment in bronchopulmonary fistula (BPF) patients the antibiotics inside the perfusate play some role in the prevention of local bacterial colonization that might lead to lethal pneumonia. The two ACC patients had pneumonia only once before the membrane stent placement and it was quickly controlled by a 1-week-long intravenous antibiotic treatment. Pneumonia did not recur during 1-year follow-up.
According to the stented aortic matrix airway reconstruction method, the implanted membrane stent was withdrawn after a 2-year follow-up. Although theoretically the membrane stent can be removed once the revascularization process is complete, we decided to keep it as a precautionary measure. With the membrane stent kept in the bronchus, no severe stent-related complications, such as bleeding, bronchitis, or pneumonia, were recorded until the 1-year follow-up.
Conclusions
To the best of our knowledge, these are the first bioengineered carina reconstruction patients who survived for more than 1 year. The “in vivo bioreactor” design provides a promising method to solve the revascularization and anti-infection problems faced during long-segment airway replacement. Prospective clinical trials are required to further prove its advantages.
Acknowledgments
Funding: This project was supported by the High-tech Three-year Action Project of Shanghai Hospital Development Center, project No. 16CR3066B. This project was also sponsored by Shanghai Science and Technology Committee, project No.17XD1424600. This project was supported by the National Natural Science Foundation of China, project No. 81871497.
Footnote
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-534
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-534
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-534). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Shanghai Chest Hospital [No. KS(Y)1744] and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Udelsman B, Mathisen DJ, Ott HC. A reassessment of tracheal substitutes-a systematic review. Ann Cardiothorac Surg 2018;7:175-82. [Crossref] [PubMed]
- Langer R, Vacanti JP. Tissue engineering. Science 1993;260:920-6. [Crossref] [PubMed]
- Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019;364:458-64. [Crossref] [PubMed]
- Martinod E, Seguin A, Radu DM, et al. Airway transplantation: a challenge for regenerative medicine. Eur J Med Res 2013;18:25. [Crossref] [PubMed]
- Tan Q, Steiner R, Hoerstrup SP, et al. Tissue-engineered trachea: History, problems and the future. Eur J Cardiothorac Surg 2006;30:782-6. [Crossref] [PubMed]
- Tan Q, Steiner R, Yang L, et al. Accelerated angiogenesis by continuous medium flow with vascular endothelial growth factor inside tissue-engineered trachea. Eur J Cardiothorac Surg 2007;31:806-11. [Crossref] [PubMed]
- Tan Q, El-Badry AM, Contaldo C, et al. The effect of perfluorocarbon-based artificial oxygen carriers on tissue-engineered trachea. Tissue Eng Part A 2009;15:2471-80. [Crossref] [PubMed]
- Tan Q, Liu R, Chen X, et al. Clinic application of tissue engineered bronchus for lung cancer treatment. J Thorac Dis 2017;9:22-9. [Crossref] [PubMed]
- Grillo HC. The history of tracheal surgery. Chest Surg Clin N Am 2003;13:175-89. [Crossref] [PubMed]
- Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med 2010;362:138-45. [Crossref] [PubMed]
- Fabre D, Kolb F, Fadel E, et al. Successful tracheal replacement in humans using autologous tissues: an 8-year experience. Ann Thorac Surg 2013;96:1146-55. [Crossref] [PubMed]
- Martinod E, Chouahnia K, Radu DM, et al. Feasibility of bioengineered tracheal and bronchial reconstruction using stented aortic matrices. JAMA 2018;319:2212-22. [Crossref] [PubMed]
- Teixeira da Silva JA. Ethical perspectives and ramifications of the Paolo Macchiarini case. Indian J Med Ethics 2017;2:270-5. [PubMed]


