Predictive and prognostic significance of M descriptors of the 8th TNM classification for advanced NSCLC patients treated with immune checkpoint inhibitors
Introduction
Stage classification aims to provide a consistent language to describe the anatomic extent of disease and is a critical tool in the management of patients with lung cancer (1). In 2015, the International Association for the Study of Lung Cancer (IASLC) collected 1,059 non-small-cell lung cancer (NSCLC) cases for a detailed analysis of the clinical M categories (2). They found that in primary M1b stage, tumor with single metastases in a single organ had significantly better prognosis than those with multiple metastases in one or several organs. Based on those findings, they proposed a new classification for M descriptors in the eighth edition of the tumor, node, and metastases (TNM) classification for lung cancer (2,3), which kept M1a category unchanged (for intrathoracic metastases including pleural/pericardial effusions, contralateral/bilateral lung nodules, contralateral/bilateral pleural nodules, or a combination of multiple of these parameters) while reclassified M1b category as single metastases lesions in a single distant organ and M1c category as multiple lesions in a single organ or multiple lesions in multiple organs. In 2017, Shin and his team (4) initiated a study involving 1,024 patients with stage IV NSCLC to validate the prognostic value of this newly proposed M descriptors in an independent cohort with multivariate and subgroup analysis. They demonstrated that a significantly shorter OS was noted for the M1b group than M1a group (HR=1.30, P=0.03) and for the M1c group than the M1b group (HR=1.57, P<0.001), regardless of pathologic and molecular subtypes, which verified the distinguishable prognostic implications. And after that, several other studies confirmed again the prognostic significance of M descriptors both in SCLC and NSCLC patients (4-6).
However, this new classification system has its own limitation as majority of patients enrolled in the analysis were treated with surgery (3,7). As we all known, most patients with lung cancer present with metastatically advanced diseases at diagnosis, which result in the lose of opportunity for surgery (8). In the last decades, therapeutic paradigms for advanced NSCLC have underwent a dramatically development (9,10). Especially, immune checkpoints inhibitors (ICIs) have shown remarkably early success in many malignancies such as melanoma, renal cell cancer as well as NSCLC (11-20). Pembrolizumab and nivolumab [programmed death 1 (PD-1) antibodies] have been approved by Food And Drug Administration (FDA) in the management of advanced NSCLC patients (11,12,14,15,21,22). However, despite the promising clinical application, the therapeutic efficiency of ICIs varies greatly among individuals (23). In unselected advanced NSCLC patients, the objective response rates (ORRs) were only 15–20% (24,25). Studies have explored a number of potential biomarkers to predict the response to ICIs such as intratumoral PD-L1 expression, lymphocytic infiltration, tumor mutation burden and neoantigens (25-29). However, with the tumor development, progress and metastasis, all those biomarkers were in dynamic and ongoing process (25,29-32). Since TNM classification aimed to describe the anatomic extent of disease and M descriptors could clearly reflect the systematic invasion status, whether it could predict the clinical outcomes of ICIs was unclear.
In this study, based on the 8th edition of TNM classification, we assessed the impact of M descriptors on the clinical outcomes of advanced NSCLC patients treated with pembrolizumab or nivolumab monotherapy.
Methods
Study population and data collection
We retrospectively screened all NSCLC patients treated in Shanghai Pulmonary Hospital from January 2012 to December 2018. Patients who met the following inclusion criteria were included: aged ≥18 years; had histologically or pathologically confirmed stage IIIB or stage IV or recurrent NSCLC; were treated with monotherapy of ICIs at least more than two cycles. Before starting immunotherapy, a complete medical history interview, physical examination, laboratory tests, and integrated staging imaging studies were available for each patient. The electric medical records were retrospectively reviewed and detailed clinicopathologic characteristics, including age, gender, smoking history, tumor histology and regime of ICIs as well as clinical response were recorded for all enrolled patients. The study was approved by the Ethics Committees of Shanghai Pulmonary Hospital Affiliated with Tongji University (No. K18-121) and was carried out in accordance with the World Medical Association’s Declaration of Helsinki (as revised in 2013).
Evaluation of M staging
M stage was determined according to the 8th edition of TNM lung cancer staging, which defines M0 as no metastases; M1a as metastases within the chest cavity, including separate tumor nodules in a contralateral lobe, pleural or pericardial nodule, or effusion; M1b as single extrathoracic metastases; and M1c as multiple extrathoracic metastases in one or more organs, based on the review of computed tomography (CT), positron emission tomography (PET)-CT scans, cranial magnetic resonance imaging (MRI), abdominal ultrasound and bone scan.
Statistical analysis
The prevalence of M1a, M1b, M1c diseases was obtained. Clinical characteristics were compared among M1a, M1b and M1c groups using chi-square test or Fisher’s exact test for categorical variables. The progress-free survival (PFS) (calculated from the first date treated with ICIs to the date of disease progression or death), overall survival (OS) (calculated from the date beginning with immunotherapy to the date of death from any cause or was censored at the last follow-up date), disease control rate (DCR) and ORR were compared according to M stages. Kaplan-Meier curves and two-sided log-rank tests were used for survival analyses. Cox proportional hazards model was used for uni- and multivariate survival analyses to calculate the hazard ratios (HR) and corresponding 95% confidence intervals (CI). All statistical analyses were performed using the SPSS statistical software, version 22.0 (SPSS Inc., Chicago, IL, USA). The survival curves were draw by GraphPad prism 7.03. P values were two-sided and considered significant if less than 0.05.
Results
Patients characteristics
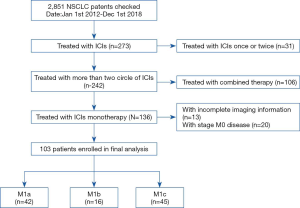
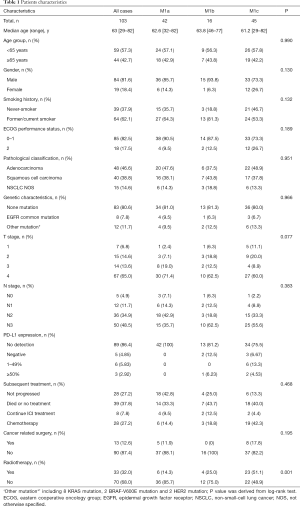
A total of 2,851 advanced NSCLC patients treated in Shanghai pulmonary hospital from January 2012 to December 2018 were screened. Among them, 242 patients were treated with more than two cycles of ICIs. In total, 103 patients received ICIs monotherapy and accompanied with distant metastases were involved eventually (Figure 1). The media age was 62 years old, 84 (81.6%) patients were man, 64 (62.1%) patients were former or current smoker, 48 (46.6%) patients had squamous cell carcinoma and 40 (38.8%) had adenocarcinoma. Among those 103 patients, 42 (34.15%) patients had M1a diseases, 16 (13.0%) had M1b diseases and 45 (36.59%) had M1c diseases. Thirteen (12.6%) patients were treated with ICIs in first-line setting, 55 (53.4%) were treated with ICIs in second-line setting and 35 (34.0%) were treated in third-line setting or later. Sixty-eight (66.0%) patients were treated with pembrolizumab and 35 (34.0%) were treated with nivolumab. PD-L1 status was available for 14 (13.6%) patients (3 patients in M1b group and 11 patients in M1c group). Using a 1% cut-off, 9 (64.3%) patients were positive. In M1b group, 2 patients had PD-L1 negative tumor and 1 had PD-L1 proportion score (TPS) more than 50% tumor. In M1c group, 3 patients had PD-L1 negative tumor, 6 had PD-L1 TPS 1–49% tumor, and 2 had PD-L1 TPS ≥50% tumor. The detailed clinical characteristics of all patients were summarized in Table 1.
Full table
Treatment outcomes
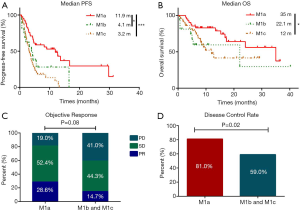
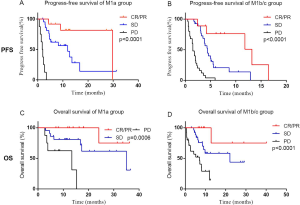
The median PFS for the whole cohort was 4.3 months. Patients with M1a disease had a significantly longer median PFS than those with M1b and M1c diseases (11.9 vs. 4.1 vs. 3.2 months, respectively, P=0.0002), while no statistically difference between M1b and M1c cohort (Figure 2A). The median OS of the whole cohort was 22.1 months. Patients with M1a diseases demonstrated a statistically longer median OS than those with M1b and M1c diseases (35 vs. 22.1 vs. 12 months, P=0.02) (Figure 2B). The ORR of M1a group showed a marginally significant superiority than M1b and M1c group (28.6% vs. 14.8%, P=0.08) (Figure 2C). The DCR of M1a group was statistically significantly higher than M1b and M1c group (81% vs. 59%, P=0.02) (Figure 2D) (Table 2).
Full table
Subgroup analysis
Subgroup analysis of PFS and OS showed a high consistent with the overall results (Figure 3). For PFS, we found that regardless of age group, sex, smoking history, genetic status, pathological type, line of ICIs or type of ICIs, patients with M1a diseases showed a significant superiority than M1b and M1c diseases except for those with ECOG PS of 2, other mutation and treated with ICIs in the first-line setting, which may account for the relatively small number of cases (Figure 3A). As for OS, the majority of subgroups favored M1a cohorts with better survival comparing with M1b and M1c cohorts. Male, smoker, under the age of 65 years and treated with nivolumab patients showed a significantly OS benefit in M1a cohort than M1b and M1c cohorts (Figure 3B).
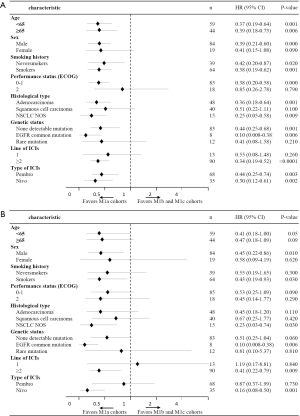
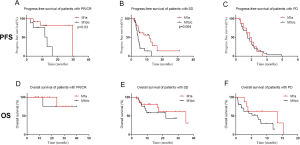
Regarding the impact of response to ICI treatment on PFS and OS, firstly, we analyzed the effects of clinical response to PFS and OS in two cohorts (M1a versus M1b/M1c). As shown in Figure S1, patients who achieved response to ICIs (CR/PR) had significantly prolonged PFS and OS than those who achieved SD or PD to ICIs, regardless of M descriptors (Figure S1A,B,C,D). Secondly, we evaluated the PFS and OS according to different clinical response (CR/PR versus SD versus PD). As shown in Figure S2, for PFS, patients in M1a group demonstrated a statistically better PFS than those in M1b/c group both in patients who achieved CR/PR or SD while no difference was found in those who achieved PD (Figure S2A,B,C). As for OS, no obvious difference was observed in CR/PR, SD or PD, which may account for the not reaching median OS and limited cases (Figure S2D,E,F). Taken together, the high ORR to ICI treatment may partially explain the better clinical outcomes of patients with M1a disease, other mechanisms may still exist.
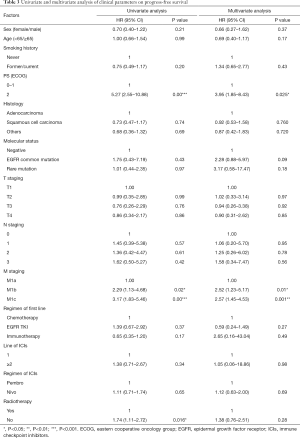
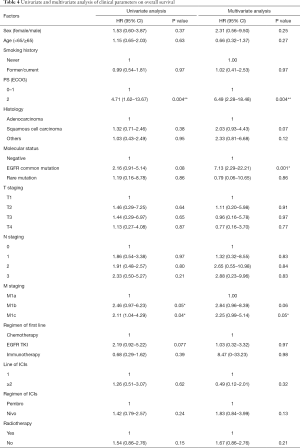
Uni- and multivariate analysis
Univariate and multivariate analysis data were presented in Tables 3 and 4. In univariate analysis, we found that worse ECOG PS, M1b and M1c status and no radiotherapy history were significantly associated with shorter PFS (Table 3). Meanwhile, M1b and M1c status as well as worse PS scores were associated with significantly shorter OS (Table 4). Multivariate analysis showed that ECOG PS of 2 was independently associated with significantly shorter PFS and OS (Tables 3,4). M1a stage was independently and significantly associated with longer PFS and OS (Tables 3,4). EGFR common mutation was associated with significantly shorter OS (P=0.001) (Table 4).
Full table
Full table
Subsequent treatment
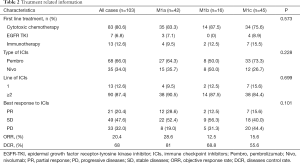
In this study, 28 (27.2%) patients have not yet progressed on ICI treatment. For the rest 75 (72.8%) patients, 19 (18.4%) patients died after immunotherapy, 8 (7.8%) patients continued ICI treatment after progression, 28 (27.2%) patients received salvage chemotherapies, and 20 (19.4%) patients did not receive any subsequent treatment. In patients with M1a, M1b, M1c disease, 23.9%, 31.3% and 46.7% received subsequent therapy. Overall, the proportions of patients who received subsequent treatment among the three groups were well balanced (Table 1).
Among 8 patients with EGFR mutation, 3 patients died after receiving one to three cycles of ICI treatment; 3 patients were still on ICI treatment; 2 patient received salvage chemotherapies (1 received docetaxel, 1 received pemetrexed plus carboplatin). No patients received TKIs as subsequent treatment after failure on ICI treatment.
Discussion
To the best of our knowledge, this was the first retrospective study to investigate the association of the revised M descriptors of the eighth edition of TNM classification with clinical outcomes of ICIs monotherapy in advanced NSCLC patients. Our study found that patients with M1a diseases had a statistically prolonged OS than M1c patients and had a numerically longer OS than M1b patients. The subgroup analysis kept high consistency with overall results as patients M1a diseases showed better survival than those M1b or M1c diseases. For PFS, patients with stage M1a diseases also had significantly longer PFS than those with M1b or M1c diseases. Notably, patients with M1b diseases also showed trends towards better PFS and OS than those with M1c diseases but lack of statistical significance, probably due to a limited sample size.
With the promotion of the new M category in the 8th edition of TNM classification, several studies have confirmed the prognostic value of M descriptors in different cohorts (4-6,33). In our study, we found that patients with M1a diseases had a statistically prolonged OS than M1c diseases (median OS: 35 vs. 12 months, P=0.02) and a numerically longer OS than M1b diseases (median OS: 35 vs. 22.1 months, P=0.08). And this superiority kept highly consistent in subgroup analysis regardless of sex, age, histological types, smoking status, and other baseline characteristics. However, our study found no significant difference when comparing M1b group with M1c group, despite a numerical superiority (median OS: 22.1 vs. 12 months, P=0.54), which may account for the limited patients in M1b cohort. Nevertheless, our study validated the prognostic value of M descriptors in advanced NSCLC patients treated with ICIs.
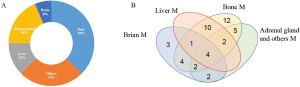
Despite the M descriptors in the 8th TNM classification demonstrated a strong correlation with the management of NSCLC patients, most studies considered OS as the main endpoints and few evaluated its impact on therapeutic outcomes. In our study, M1a diseases were significantly associated with prolonged PFS comparing with M1b and M1c diseases. Several factors may explain the findings. Firstly, evidence suggested that patients with baseline liver metastases have been shown to have minimal therapeutic benefit with ICI treatment (34-39). Recently, the 3-year update and outcomes in patients with liver metastases from pooled CheckMate 017 and CheckMate 057 found that median OS for all randomized pooled patients was 11.1 months while only 6.8 months for those with liver metastases (40). Furthermore, two clinical trials (Study 1108 and ATLANTIC) also demonstrated that liver metastasis was an independent negative prognostic factor for OS and PFS (38,41). One possible explanation for the poor outcomes among ICI-treated NSCLC patients with liver metastasis was the tendency for poorer PS and severe hepatic side effects compare to those without (36,37,39). In our study, M1a cohort had no extrathoracic metastases while 12% of patients in M1b cohort had liver metastases, and for M1c cohort, almost half of patients had liver metastases (Figure S3), which may greatly decrease the survival benefits with ICI treatment. Secondly, a higher proportion of patients with M1c diseases had ECOG PS of 2 than M1a and M1b diseases (26.7% vs. 9.5% vs. 12.5%, respectively). Univariate and multivariate analysis showed that ECOG PS of 2 was significantly associated with shorter PFS and OS. Patients with NSCLC with ECOG PS of 2 were a heterogeneous group, usually with a large tumor burden in conjunction with chronic obstructive pulmonary disease (COPD) or other smoking-related diseases, necessitating frequent treatment with corticosteroids (42). A phase 2 trial (CheckMate 171) of nivolumab in previously treated advanced squamous NSCLC involved 103 patients with ECOG PS 2. The median OS in ECOG PS 2 patient population was 5.2 months versus 10.0 months in the overall population (43), which was consistent with findings from another prospective 3B/4 study (CheckMate 153) of nivolumab in advanced NSCLC (44). Thirdly, Oh and his team (45) found that it was extra-thoracic tumor burden rather than thoracic tumor burden that was negatively correlated with clinical outcomes for patients with extensive stage small cell lung cancer, which was similar with our findings that patients with extra-thoracic metastases showed worse PFS than those with intro-thoracic metastases.
Several limitations should be taken into consideration. Firstly, this was a retrospective study and selection bias cannot be avoided. Secondly, the patients enrolled in the final analysis was relatively small, especially in M1b cohort. Thirdly, to date, biomarkers reflecting the clinical response to ICIs were still unclear, studies suggested that high expression of PD-L1 in tumors (16,27), mismatch repair (MMR) deficient (46) and high tumor mutation burden (14,47-49) were associated with an improved outcomes in patients treated with immunotherapy. What a regret was that only a few patients enrolled in this study have been tested for those biomarkers, so we could not analyze the association of these biomarkers with different metastases status.
Conclusions
In conclusion, the present study demonstrated that M descriptors of the 8th TNM classification had a clear association with the therapeutic response to ICIs and it had a significantly prognostic value in advanced NSCLC patients treated with ICI treatment. M descriptors may need to be considered in future prospective studies.
Acknowledgments
Funding: This study was supported in part by grants from the National Science Foundation of China (No. 81703020, 81871865).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-19-396). TJ serves as an unpaid editorial board member of Translational Lung Cancer Research from Feb 2018 to Jan 2021. YH serves as an unpaid editorial board member of Translational Lung Cancer Research from Jan 2020 to Dec 2021. CZ serves as an unpaid editorial board member of Translational Lung Cancer Research from Mar 2012 to Mar 2022. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committees of Shanghai Pulmonary Hospital Affiliated with Tongji University (No. K18-121) and was carried out in accordance with the World Medical Association’s Declaration of Helsinki (as revised in 2013). Informed consent was waived due to the retrospective nature of the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Chansky K, Groome P, et al. The IASLC Lung Cancer Staging Project: Methodology and Validation Used in the Development of Proposals for Revision of the Stage Classification of NSCLC in the Forthcoming (Eighth) Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1433-46. [Crossref] [PubMed]
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Shin J, Keam B, Kim M, et al. Prognostic Impact of Newly Proposed M Descriptors in TNM Classification of Non–Small Cell Lung Cancer. J Thorac Oncol 2017;12:520-8. [Crossref] [PubMed]
- Shirasawa M, Fukui T, Kusuhara S, et al. Prognostic significance of the 8th edition of the TNM classification for patients with extensive disease small cell lung cancer. Cancer Manag Res 2018;10:6039-47.
- Tendler S, Grozman V, Lewensohn R, et al. Validation of the 8th TNM classification for small-cell lung cancer in a retrospective material from Sweden. Lung Cancer 2018;120:75-81. [Crossref] [PubMed]
- Choi HS, Jeong BK, Jeong H, et al. Application of the new 8th TNM staging system for non-small cell lung cancer: treated with curative concurrent chemoradiotherapy. Radiat Oncol 2017;12:122. [Crossref] [PubMed]
- Le L, van Dams R, Lee P. Is first-line pembrolizumab appropriate for all patients with metastatic non-squamous histology non-small cell lung cancer patients? Transl Lung Cancer Res 2019;8:S327-30. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Fiorelli A, Vitiello F, Morgillo F, et al. Pembrolizumab monotherapy in advanced NSCLC patients with low PD-L1 expression: is there real evidence? Transl Cancer Res 2019;8:S618-20. [Crossref]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Afzal MZ, Dragnev KH, Shirai K. An extended overall survival analysis of pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced non-squamous non-small cell lung cancer. Ann Transl Med 2019;7:S53. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Forde PM, Kelly RJ, Brahmer JR. New strategies in lung cancer: translating immunotherapy into clinical practice. Clin Cancer Res 2014;20:1067-73. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Rina H, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018. [Crossref] [PubMed]
- Pai-Scherf L, Blumenthal GM, Li H, et al. FDA Approval Summary: Pembrolizumab for Treatment of Metastatic Non-Small Cell Lung Cancer: First-Line Therapy and Beyond. Oncologist 2017;22:theoncologist.2017-0078.
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Shukuya T, Carbone DP. Predictive Markers for the Efficacy of Anti-PD-1/PD-L1 Antibodies in Lung Cancer. J Thorac Oncol 2016;11:976-88. [Crossref] [PubMed]
- Tang H, Wang Y, Chlewicki LK, et al. Facilitating T Cell Infiltration in Tumor Microenvironment Overcomes Resistance to PD-L1 Blockade. Cancer Cell 2016;29:285-96. [Crossref] [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014;20:5064-74. [Crossref] [PubMed]
- Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther 2015;14:847-56. [Crossref] [PubMed]
- Martinez P, Peters S, Stammers T, et al. Immunotherapy for the First-Line Treatment of Patients with Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res 2019;25:2691-8. [Crossref] [PubMed]
- Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med 2017;15:133. [Crossref] [PubMed]
- Blomberg OS, Spagnuolo L, de Visser KE. Immune regulation of metastasis: mechanistic insights and therapeutic opportunities. Dis Model Mech 2018.11. [PubMed]
- Zhang J, Fujimoto J, Zhang J, et al. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science 2014;346:256-9. [Crossref] [PubMed]
- Nieder C, Hintz M, Oehlke O, et al. The TNM 8 M1b and M1c classification for non-small cell lung cancer in a cohort of patients with brain metastases. Clin Transl Oncol 2017;19:1141-6. [Crossref] [PubMed]
- Ahn BC, Pyo KH, Xin CF, et al. Comprehensive analysis of the characteristics and treatment outcomes of patients with non-small cell lung cancer treated with anti-PD-1 therapy in real-world practice. J Cancer Res Clin Oncol 2019;145:1613-23. [Crossref] [PubMed]
- Funazo T, Nomizo T, Kim YH. Liver Metastasis Is Associated with Poor Progression-Free Survival in Patients with Non–Small Cell Lung Cancer Treated with Nivolumab. J Thorac Oncol 2017;12:e140-1. [Crossref] [PubMed]
- Kitadai R, Okuma Y, Hakozaki T, et al. The efficacy of immune checkpoint inhibitors in advanced non-small-cell lung cancer with liver metastases. J Cancer Res Clin Oncol 2020;146:777-85. [Crossref] [PubMed]
- Qin BD, Jiao XD, Liu J, et al. The effect of liver metastasis on efficacy of immunotherapy plus chemotherapy in advanced lung cancer. Crit Rev Oncol Hematol 2020;147:102893. [Crossref] [PubMed]
- Sridhar S, Paz-Ares L, Liu H, et al. Prognostic Significance of Liver Metastasis in Durvalumab-Treated Lung Cancer Patients. Clin Lung Cancer 2019;20:e601-8. [Crossref] [PubMed]
- Tumeh PC, Hellmann MD, Hamid O, et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol Res 2017;5:417-24. [Crossref] [PubMed]
- Vokes EE, Ready N, Felip E, et al. Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 2018;29:959-65. [Crossref] [PubMed]
- Garassino MC, Cho BC, Kim JH, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018;19:521-36. [Crossref] [PubMed]
- Passaro A, Spitaleri G, Gyawali B, et al. Immunotherapy in Non–Small-Cell Lung Cancer Patients With Performance Status 2: Clinical Decision Making With Scant Evidence. J Clin Oncol 2019;37:1863-7. [Crossref] [PubMed]
- Felip E, Ardizzoni A, Ciuleanu T, et al. CheckMate 171: A phase 2 trial of nivolumab in patients with previously treated advanced squamous non-small cell lung cancer, including ECOG PS 2 and elderly populations. Eur J Cancer 2020;127:160-72. [Crossref] [PubMed]
- Spigel DR, McCleod M, Jotte RM, et al. Safety, Efficacy, and Patient-Reported Health-Related Quality of Life and Symptom Burden with Nivolumab in Patients with Advanced Non–Small Cell Lung Cancer, Including Patients Aged 70 Years or Older or with Poor Performance Status (CheckMate 153). J Thorac Oncol 2019;14:1628-39. [Crossref] [PubMed]
- Oh JR, Seo JH, Hong CM, et al. Extra-thoracic tumor burden but not thoracic tumor burden on (18)F-FDG PET/CT is an independent prognostic biomarker for extensive-disease small cell lung cancer. Lung Cancer 2013;81:218-25. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Garber K. Blood test may predict cancer immunotherapy benefit. Science 2018;360:1387. [Crossref] [PubMed]
- Hellmann MD, Nathanson T, Rizvi H, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018;33:843-52.e4. [Crossref] [PubMed]
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018;33:853-61.e4. [Crossref] [PubMed]