
How to select the best upfront therapy for metastatic disease? Focus on ALK-rearranged non-small cell lung cancer (NSCLC)
Introduction
Lung cancer is the most common cause of cancer-related death in both men and women worldwide (1). About 4–6% of non-small cell lung cancers (NSCLCs) develop an inversion on the short arm of chromosome 2 that joins the 5' end of the echinoderm microtubule-associated protein-like 4 (EML4) with the 3' end of the anaplastic lymphoma kinase (ALK) gene, which results in a chimeric protein EML4-ALK (2,3).
EML4-ALK fusions have distinct clinical characteristics and most commonly seen in patients with adenocarcinoma, young age, and a never/light smoking history (4).
The EML4-ALK fusion can be detected by fluorescent in situ hybridization (FISH), immunohistochemistry (IHC), or next generation sequencing (NGS) and are very sensitive to treatment of ALK tyrosine kinase inhibitors (TKIs). With NGS, at least 90 distinct fusion partners have been identified in ALK-rearranged NSCLC (5).
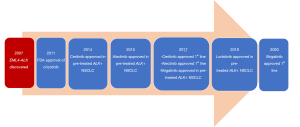
After the EML4-ALK translocation was first discovered in a lung cancer cell line in 2007, crizotinib, a first-generation ALK inhibitor was approved merely 4 years later. A timeline of key events in the development of first-line ALK TKIs is shown in Figure 1.
Since then, second-generation ALK inhibitor, ceritinib has been shown to be superior to chemotherapy while alectinib and brigatinib have demonstrated superior efficacy and better CNS activity compared to crizotinib (6-8). Third-generation lorlatinib has now demonstrated efficacy in the treatment naïve setting and after resistance to first and second-generation of ALK TKIs (9). In the US, crizotinib, alectinib, brigatinib,and ceritinib are ALK TKIs approved in the first-line. Ceritinib, brigatinib, and lorlatinib have been approved for second or in further lines of therapy. Progression free survival (PFS), response rate, and intracranial activity of crizotinib, ceritinib, alectinib, brigatinib, lorlatinib, and ensartinib is summarized in Table 1.

Full table
Comparison of median PFS among phase 3 ALK TKI trials is shown in Figure 2.
Overview of ALK TKIs
Crizotinib
Crizotinib was compared to chemotherapy in a phase 3 PROFILE 1014 trial comparing crizotinib 250 mg twice daily to chemotherapy with carboplatin/cisplatin plus pemetrexed in patients with ALK-rearranged nonsquamous NSCLC. Crizotinib was found to have superior response rate and PFS compared to chemotherapy and better quality of life. The median PFS of the crizotinib group was 10.9 vs. 7.9 months in the chemotherapy arm [hazard ratio (HR) for progression or death with crizotinib, 0.45; 95% confidence interval (CI), 0.35–0.60; P<0.001]. Objective response rate of crizotinib was 74% compared to 45% with chemotherapy (P<0.001) (10). Four-year overall survival (OS) was 56.6% for crizotinib and 49.1% for chemotherapy. HR for death with crizotinib was not significantly different than with chemotherapy, 0.760; 95% CI, 0.548–1.052 (P=0.0978) (15). The most common toxicities of crizotinib included vision disorder (71%), diarrhea (61%), and edema (49%). A quality of life assessment was performed on this study and found that patients treated with crizotinib, compared to chemotherapy, had decreased lung cancer symptoms and improved quality of life (10).
Ceritinib
The efficacy of ceritinib was shown in a phase 3 ASCEND-4 open-label randomized trial that compared oral ceritinib 750 mg/day compared to platinum-pemetrexed chemotherapy for four cycles followed by maintenance pemetrexed. Ceritinib demonstrated superior PFS compared to chemotherapy with median PFS of 16.6 (95% CI, 12.6–27.2) months in the ceritinib group vs. 8.1 (95% CI, 5.8–11.1) months in the chemotherapy group. Overall response rate (ORR) in the ceritinib group was 72.5% (95% CI, 65.5–78.7%) compared to 26.7% (95% CI, 20.5–33.7%) in the chemotherapy group. Ceritinib was demonstrated to have CNS activity with a CNS response rate of 72.7% (95% CI, 49.8–89.3%) in patients with measurable baseline brain metastases. The most common side effects of ceritinib were gastrointestinal related symptoms including diarrhea (85%), nausea (69%), and vomiting (66%). However, the toxicity profile of ceritinib was rather significant with the dose decreases or interruptions rates to be 80% with ceritinib compared to 45% with chemotherapy (11). A later trial concluded that ceritinib at 450 mg taken with food had similar activity to 750 mg taken fasting and reduced gastrointestinal toxicities (16).
Alectinib
Alectinib has demonstrated efficacy in the first-line setting in the treatment of ALK-rearranged lung cancer in the phase III ALEX study (7). This trial compared alectinib 600 mg twice daily with crizotinib 250 mg twice daily in treatment naïve patients. Alectinib showed better efficacy with superior 12-month event free survival, PFS, and decreased CNS progression. The ORR was higher in the alectinib group (82.9%; 95% CI, 76.0–88.5%) compared to crizotinib (75.5%; 95% CI, 67.8–82.1%) but did not reach statistical significance (P=0.09). The 12-month event free survival was significantly longer with alectinib, 68.4% (95% CI, 61.0–75.9%) compared with crizotinib 48.7% (95% CI, 40.4–56.9%); HR for PFS, 0.47 (95% CI, 0.34–0.65); P<0.001. CNS disease was better controlled with alectinib than with crizotinib. CNS progression occurred in 18 patients (12%) in the alectinib group compared to 68 patients (45%) in the crizotinib group (HR, 0.16; 95% CI, 0.10–0.28; P<0.001) (7). Updated data from the ALEX trial showed a median investigator-assessed PFS of 34.8 [95% CI, 17.7–not reached (NR)] months in the alectinib group compared to 10.9 (95% CI, 9.1–12.9) months with crizotinib (12). Five-year survival rate was 62.5% (95% CI, 54.3–70.8%) with alectinib vs. 45.5% (95% CI, 33.6–57.4%) with crizotinib although the OS data remains (17).
The alectinib group experienced less grade 3 to 5 toxicities, 41% with alectinib versus 50% with crizotinib. Any grade adverse events were similar between the two groups with 97% of patients in both groups experiencing any adverse even. The most common side effects in the crizotinib group were gastrointestinal symptoms including nausea (48%), diarrhea (45%), and vomiting (38%). Patients in the alectinib group most commonly experienced peripheral edema (17%), anemia (20%) and increases in liver function tests including elevations in ALT (15%), AST (14%), and bilirubin (15%).
Alectinib was also studied in the J-ALEX and ALESIA trials (18,19). The J-ALEX was a randomized phase III study that evaluated crizotinib versus alectinib in ALK inhibitor naïve Japanese patients. Alectinib again showed superior PFS compared to crizotinib with median PFS NR in the alectinib arm (95% CI, 20.3–not estimated) and 10.2 months in the crizotinib arm (95% CI, 8.2–12.0); HR, 0.34 (99.7% CI, 0.17–0.71); P<0.0001. Objective response rate was also higher with alectinib compared to crizotinib 92% (95% CI, 85.6–97.5%) and 79% (95% CI, 70.5–87.3%) respectively (18). The ALESIA trial also demonstrated improved PFS of alectinib compared to crizotinib in the Asian patients which included investigational sites in China, South Korea, and Thailand. PFS was significantly better with alectinib compared to crizotinib. Median PFS was NR in the alectinib group compared with 11.1 months in the crizotinib group (HR, 0.22; 95% CI, 0.13–0.38; P<0.0001). Again, alectinib was shown to have better objective response rate compared to crizotinib, 91% vs. 77%, and longer duration of response (HR, 0.22; 95% CI, 0.12–0.40; P<0.0001). CNS response was also better with alectinib 73% compared to 22% with crizotinib (19).
The BFAST study evaluated the utility of stand-alone blood-based NGS in biomarker detection. In one arm of this study, clinical activity of alectinib was evaluated in those who had ALK positivity detected by blood-based biomarker testing alone. Treatment with alectinib resulted in an investigator-confirmed ORR of 87.4% and PFS of 78.38% in those who were ALK+ by blood-based NGS, demonstrating the effectiveness of using blood-based NGS in biomarker detection and clinical decision making (20).
Brigatinib
A second-generation ALK inhibitor, brigatinib, was developed along with alectinib and ceritinib for patients who had progressed on crizotinib. Brigatinib, in the first-line setting, was studied in the ALTA-1L trial that randomized patients with treatment naïve ALK-rearranged NSCLC to brigatinib 180 mg once daily (with 90 mg daily for an initial 7-day lead-in period) compared to crizotinib at 250 mg twice daily. Brigatinib was found to have superior efficacy compared to crizotinib with better PFS and intracranial response. The estimated 12-month PFS was 67% (95% CI, 56–75%) with brigatinib compared with 43% with crizotinib (95% CI, 32–53); HR for disease progression or death, 0.49 (95% CI, 0.33–0.74); P<0.001. The ORR was numerically higher in the brigatinib group 71% (95% CI, 62–78%) compared to crizotinib 60% (95% CI, 51–68%), odds ratio 1.59 (95% CI, 0.96–2.62). Intracranial response rates were significantly higher in the brigatinib group than crizotinib group at 78% (95% CI, 52–94%) and 29% (95% CI, 11–52%), respectively, odds ratio 10.42 (95% CI, 1.90–57.05) (8). Updated data from the ALTA-1L study showed blinded independent review committee (BIRC) PFS of 24.0 (95% CI, 18.5–NR) months with brigatinib compared to 11.0 (95% CI, 9.2–12.9) months with crizotinib. Investigator-assessed PFS demonstrated median PFS of 29.4 (95% CI, 21.2–NR) months with brigatinib vs. 9.2 (95% CI, 7.4–12.9) months with crizotinib. Intracranial PFS by BIRC was significantly better in the brigatinib group compared to crizotinib, 24 (95% CI, 12.9–NR) months compared to 5.5 (95% CI, 3.7–7.5) months respectively (13). However, the efficacy of brigatinib in alectinib-refractory disease demonstrated limited activity, with an ORR of 17% and median PFS of 4.4 months (21).
Adverse events were similar between the brigatinib and crizotinib groups with 97% of the brigatinib patients and 100% of the crizotinib patients experiencing any grade adverse event. The most common side effects in the brigatinib group included diarrhea (49%), increased creatine kinase level (39%), and nausea (26%). The most common side effect of the crizotinib group included nausea (56%), diarrhea (55%) and vomiting (39%) (8).
Lorlatinib
Lorlatinib is a reversible potent third-generation TKI that is highly selective and targets ALK and ROS1 kinase domain. It was developed to overcome resistant ALK mutants including the common G1202R. Lorlatinib has excellent penetration to the blood brain barrier and its efficacy has also been demonstrated even in patients with intracranial metastases after progression on second-generation ALK TKIs.
In a previously reported phase I portion of a phase I–II study, the ORR with lorlatinib was objective 45% (19 out of 41) and durable responses with the median duration of response of 12.4 (95% CI, 6.5–NR) months in ALK-positive group. Many of these patients had received multiple lines of therapy and had intracranial metastases. Responses were seen in those previously treated with second-generation ALK TKIs and those who had only received crizotinib (22).
Lorlatinib received FDA accelerated approval on November 2, 2018 for the treatment of advanced ALK-rearranged NSCLC after progression on crizotinib followed by at least one other ALK TKI, or those whose disease have progressed on alectinib or ceritinib as the first ALK inhibitor (23). The approval was based on ORR, intracranial response rate and duration of response with lorlatinib in a subgroup of 215 patients in a phase II study (NCT01970865) enrolling patients who had ALK-rearranged NSCLC who had progressed on at least one line of ALK TKI. In this study, lorlatinib demonstrated efficacy and CNS activity in all groups including treatment naïve and prior treated patients up to three prior lines of therapy. The ORR in the ALK cohort was 90% (95% CI, 73.5–97.9%) in treatment naïve patients, 69.5% (95% CI, 56.1–80.8%) in those who received previous crizotinib, 32.1% (95% CI, 15.9–52.4%) in those who received a non-crizotinib TKI, and 38.7% (95% CI, 29.6–48.5%) in those who received two or more ALK TKIs. The estimated median duration of response was 12.5 months.
Intracranial ORR was 60% with complete response in 21% and an estimated median duration of intracranial response of 19.5 months. Intracranial response was observed across all cohorts, including 66.7% (95% CI, 9.4–99.2%) in treatment native patients, 87.0% (95% CI, 66.2–97.2%) in those who received previous crizotinib, 55.6% (95% CI, 21.2–86.3%) in those who received a non-crizotinib TKI, and 53.1% (95% CI, 38.3–67.5%) in those who received two or more ALK TKIs (9).
The most common adverse events observed were hypercholesterolemia (66%), hypertriglyceridemia (45%), and edema (41%). Grade 4 toxicity was rare with 1% hypercholesterolemia, 3% hypertriglyceridemia, and <1% elevated lipase, acute respiratory failure, and elevation of blood potassium (9).
Ensartinib
Ensartinib was evaluated in a phase I/II clinical trial in ALK-rearranged lung cancer patients who were treatment naïve, treated with prior ALK TKI, and brain metastases. In all ALK-positive patients treated with ≥200 mg, the response rate was 60% and median PFS was 9.2 months. In the treatment native population, the response rate was 80% and the median PFS was 26.2 months whereas the response rate was 69% and PFS was 9.0 months in patients previously treated with crizotinib alone. Intracranial response rate was 64% (14). In alectinib-refractory disease, ensartinib demonstrated a RR of 23% and a disease control rate (DCR) of 50% (24). The most common treatment related toxicities reported for ensartinib includes rash reported in 56%, nausea in 36%, pruritis in 28%, vomiting in 26% and fatigue in 22% of patients (14). The eXalt3 trial is an on-going randomized phase III trial (NCT02767804) that is comparing ensartinib with crizotinib in the first-line setting.
Selection of ALK inhibitors
For the most part, targeting ALK-rearranged NSCLC has been a therapeutic success with multiple drugs approved. However, these patients ultimately progress with on- target (ALK-dependent) or off-target (ALK-independent) resistance mechanisms (25-28). Therefore, the most optimal sequencing of the ALK inhibitors remains to be an ongoing field of further investigation. The selection of an ALK TKI is based on factors including systemic and CNS activity of the ALK TKI, various EML4-ALK variants, mechanisms of resistance as well as the toxicity profile.
In a newly diagnosed, advanced ALK-rearranged NSCLC, alectinib is generally recommended as frontline therapy due to its superiority in PFS (7) and CNS activity when compared to crizotinib (29). Brigatinib has also emerged as a potential option in the frontline setting after demonstrating favorable responses over crizotinib in the phase III ATLA-1L trial although it has a rare but real risk of early-onset pulmonary toxicity that requires close monitoring (30).
While alectinib is generally recommended as frontline therapy in the US due to its superiority in PFS and CNS activity when compared to crizotinib (7,29), crizotinib remains an option as first-line treatment in other regions of the world (31). Although some studies have shown the potential OS benefit of sequential use of a second-generation ALK inhibitor post crizotinib, these were retrospective evaluations (32-35). The updated analysis of ALEX suggests the OS benefit with first-line alectinib use as seen from the 5-year survival rate of 62.5% (95% CI, 54.3–70.8%) with alectinib vs. 45.5% (95% CI, 33.6–57.4%) with crizotinib although the OS data remains immature and the median OS with alectinib was not estimable (17). Indeed, previous studies have suggested that initial use of a second-generation ALK TKI may be superior to sequential treatment with first-generation followed by second-generation TKIs (7,8,11,18). However, in a situation where crizotinib was given as the first-line of therapy, second-generation ALK TKIs, for example, ceritinib, alectinib, brigatinib, and ensartinib, have all shown efficacy in those patients who have progressed on crizotinib (36).
Whether administered directly after first-line second-generation ALK TKI, or after sequencing with crizotonib followed by second-generation ALK TKI, the use of the third-generation ALK TKI, lorlatinib, has been FDA approved and has been generally accepted for use in this setting, although the use of stereotactic body radiation therapy (SBRT) or other local procedures such as ablation, cryotherapy or even resection maybe be considered through careful multidisciplinary discussion for oligometastatic disease progression with the continuation of the ALK inhibitors that was being used at that time (31,37).
Also important to note is that platinum doublet chemotherapy is a valid treatment option for patients with ALK translocation (38). While late generation ALK TKIs may be useful in targeting resistant on-target (ALK-dependent) mutations in ALK (for example, lorlatinib for G1202R), off-target resistance (ALK-independent) may be better treated with platinum doublet chemotherapy (31,37). Chemotherapy may be also considered for scenarios where multiple sites of progression is seen. In theory, in a situation where the progression of disease includes the brain, consideration should be given to utilize an ALK TKI in combination with chemotherapy. Since this strategy is supported mainly through retrospective observations (38), additional evidence from prospective clinical trials would be important to document the effects of combination as well as its safety.
In a scenario where both lorlatinib is not available and platinum doublet chemotherapy is not an option (or had already been given), there are data to support the use of some second-generation ALK inhibitors after failing a second-generation ALK TKI such as alectinib. The prospective phase II ASCEND-9 trial assessed the efficacy of ceritinib in patients with ALK-rearranged NSCLC who had disease progression on alectinib. This showed that the efficacy of ceritinib after alectinib was limited with an ORR of 25% (95% CI, 8.7–49.1%) and a DCR of 70% (95% CI, 45.7–88.1%) but with a median PFS of only 3.7 (95% CI, 1.9–5.3) months (39). The use of brigatinib in alectinib-refractory ALK-rearranged NSCLC patients was also assessed and showed limited activity, with an ORR in 17% of patients with measurable disease and median PFS of 4.4 months in a retrospective study (21). Ensartinib was also assessed in this setting and showed an ORR of 23% and a DCR of 50% (24).
Activity on various EML4-ALK variants
Several EML4-ALK variants have been previously reported. The most common are variant 1 (33%), where exon 13 of EML4 is fused to exon 20 of ALK (E13; A20). In variant 2 (10%), exon 20 of EML4 is fused to exon 20 of ALK (E20; A20), and in variant 3a/b (29%), exon 6a or 6b of EML4 is fused to exon 20 of ALK (E6a/b; A20) (40-42). In a Japanese study retrospectively evaluating the ALK variants of 35 patients treated with crizotinib, the most common was variant 1 in 19 patients (54%), followed by variant 2 in 5 patients (14%), variant 3a/3b in 4 patients (12%), and other variants in 7 patients (20%).
The ORR was 69% in all patients, whereas it was 74% and 63% in the variant 1 and non-variant 1 groups, respectively. The median PFS time was significantly longer in patients with variant 1 than in those with non-variant 1 [median PFS, 11.0 (95% CI, 6.5–43.0) vs. 4.2 (95% CI, 1.6–10.2) months, respectively; P=0.05]. Multivariable analysis determined two factors that were significantly associated with PFS duration which were having an ALK variant 1 (HR, 0.350; 95% CI, 0.128–0.929; P=0.05) and advanced stage (HR, 4.646; 95% CI, 1.381–21.750; P=0.05) suggesting the better efficacy of crizotinib in patients with ALK variant 1 versus non-variant 1 (43).
IHC/FISH status
ALK rearrangements can be detected by IHC, FISH or NGS. However, whether to treat with an ALK TKI in cases of discordant IHC and FISH results remain a challenge. Data collected from the ALEX trial was further evaluated to examine clinical outcomes for IHC-positive/FISH-negative NSCLC patients treated with alectinib and crizotinib. IHC testing was performed using the VENTANA ALK (D5F3) CDx Assay and FISH testing was done with the Vysis ALK Break Apart FISH Probe Kit. In the ALEX trial, 303 patients with ALK IHC positivity were randomized to receive either alectinib or crizotinib treatment. Two hundred and three patients also were found to ALK FISH positive and 39 patients were found to be ALK FISH-negative. Sixty-one of the ALK IHC-positive patients did not have a corresponding FISH result due to uninformative FISH testing or inadequate tissue for testing. Exploratory analysis revealed that PFS of ALK FISH-positive patients were similar to those of the IHC-positive patients. The ALK IHC-positive/FISH-negative patients were found to derive clinical benefit from ALK TKI indicating that ALK IHC testing may identify more patients who are eligible for ALK TKI (44).
Intracranial activity
Brain metastasis is relatively common in ALK-rearranged NSCLC patients and has been reported in approximately 20–30% of patients at initial diagnosis (45), and approximately 50% of patients will later develop CNS metastasis over the course of their disease (46). Crizotinib has limited CNS penetration due to a low CSF-to-plasma concentration ratio (0.0026) (47) which explains the reason why CNS is a common site of disease progression with crizotinib failure (45).
If the brain is the only site of progression, the use of later generation ALK inhibitors should be first considered (if not already utilized) and while radiation [stereotactic radiosurgery (SRS) or whole brain radiation (WBRT)] or surgical resection could be performed in select cases in those with large volume of disease and or those with symptomatic brain metastases. Typically, ALK TKIs are usually withheld on the days radiation therapy is being given and resumed after its completion to minimize the risk of potential neurotoxicity (48). It is important to note that the high CNS activity of alectinib, brigatinib and lorlatinib may allow clinicians to avoid the use of radiation in patients with asymptomatic CNS metastasis as there are reports of increased risk of radiation necrosis with concomitant use of ALK TKIs and cranial irradiation (49-51).
Resistance mutations
Initial responses to ALK inhibitors are not always durable, and eventually, virtually all patients develop tumor progression. Resistance can occur as on-target alterations such as ALK gene mutations or amplifications, and off-target events such as upregulation of bypass signaling pathways.
Molecular profiling at the time of diagnosis and also at the time of progression may assist in the sequential treatment selection of an ALK TKI, as specific ALK resistance mutations may predict for sensitivity to certain ALK TKIs. While a positive liquid biopsy is often times sufficient to initiate targeted therapy (52), tissue biopsy should be encouraged in cases where liquid biopsy testing does not reveal the resistance mechanism and when rapid progression appears suspicious for small cell lung cancer transformation.
The most common secondary resistant ALK mutant in patients post progression with second-generation ALK inhibitors is the Gly1202Arg (G1202R), which has been reported to occur in 21% of patients treated with ceritinib, 29% of patients on alectinib, and 43% of patients on brigatinib (25). The amino acid substitution of G1202R resides at the solvent-exposed region of ALK, where the bulkier, charged side chain is considered to cause steric interference with the binding of most ALK TKIs (25).
Specific ALK fusion variants have demonstrated improved clinical outcome. For example, having the EML4-ALK variant 3 was significantly associated with developing ALK resistance mutations, particularly at G1202R.
An exploratory analysis of 29 patients who received lorlatinib demonstrated that those with variant 3 had a significantly longer median PFS than those with variant 1 (11.0 vs. 3.3 months; P=0.011). Therefore, this EML4-ALK variant 3 could represent a potential biomarker for response to lorlatinib (26).
The approval of lorlatinib was based on a phase II study with 215 ALK-rearranged NSCLC patients who had failed at least one line of ALK inhibitors. Among patients who failed two or more ALK TKIs (including ceritinib and alectinib), ORR was 39% and median PFS was 6.9 months (9). In a subsequent analysis of tissue and plasma genotyping, those with mutations in the plasma (62%) or tissue (69%) showed higher responses to lorlatinib than those without mutations in the plasma (32%) or tissue (7%) (27), confirming the clinical utility of lorlatinib for on-target (ALK-dependent) resistance post early generation ALK inhibitors.
Lorlatinib has demonstrated efficacy in patients who were resistant to first and or second-generation ALK TKIs. However, acquired resistance to lorlatinib is unfortunately inevitable. Recently, the mechanisms of lorlatinib resistance have been characterized by sequencing lorlatinib-resistant biopsy samples from 20 patients. The dominant resistance mechanism to lorlatinib was primarily from multiple different compound ALK mutations, or, a double mutation. For example, patients harboring ALK C1156Y may develop resistance to lorlatinib by acquiring ALK C1156Y/L1198F (53). Of interest, Shaw et al. has demonstrated that the double mutation of ALK C1156Y/L1198F found at lorlatinib-resistance surprisingly restored its sensitivity to crizotinib, although the patient had previously failed crizotinib and ceritinib (54). This mimics the observation found in EGFR mutated patients who develop osimertinib third-generation EGFR TKI resistance through the C797S mutation, which has the potential to be overcome by gefitinib or erlotinib, both are first-generation EGFR TKIs.
In alectinib-refractory patients, brigatinib may represent a viable therapeutic option with those with tumors demonstrating I1171X or V1180L, but may not be as effective otherwise, including those with G1202R (21).
Molecular profiling may not need to be from tissue. Interestingly, a recent study reported by Horn et al., demonstrated the utility of circulating tumor DNA monitoring to analyze therapeutic response and resistance to ensartinib. In this study, patients with an EML4-ALK variant 1 (V1) fusion had improved response (9 of 17 patients; 53%) to ensartinib compared to patients with EML4-ALK variant 3 (V3) fusion (1 of 7 patients; 14%) (28).
Toxicities
While ALK inhibitors are generally better tolerated than chemotherapy, various toxicities have been reported. Vision disorder (71%), diarrhea (61%) and edema (49%) were the most common adverse events reported with crizotinib use from the first-line study (10). In clinical practice, nausea and vomiting can often be attributed to crizotinib as well as ceritinib. The gastrointestinal toxicities of ceritinib were rather significant in the ASCEND-4 trial comparing ceritinib to chemotherapy. Indeed, dose decreases and interruption rates were higher in the ceritinib group (80%) than in the chemotherapy arm (45%) (11). Subsequent studies have shown better tolerance of ceritinib taken with food which is what is recommended on the current drug label (16). In the ALEX study, laboratory abnormalities such as anemia and liver enzyme alterations were the most commonly reported adverse events from alectinib (7). Clinically, alectinib has been associated with edema and myalgias whereas early-onset pulmonary toxicity has been reported with brigatinib. In the ALTA-1L trial, grade 3 or 4 pulmonary toxicity of interstitial lung disease or pneumonitis was reported in 3% of patients treated with brigatinib and 0.7% of those receiving crizotinib (8). All of the four events of interstitial lung disease or pneumonitis in the brigatinib arm were “early onset” as defined as manifesting within 14 days after the start of treatment and suggests the importance of close follow up during this critical time. Other adverse events reported with the use of brigatinib included diarrhea, nausea, increased blood creatinine kinase levels and increase in lipase levels.
The unique toxicity profile of lorlatinib is also something to note. Common adverse events include hyperlipidemia and many patients require the introduction or titration of statin therapy. Of note, pravastatin, rosuvastatin or pitavastatin should be initially considered due to their lower involvement with specific CYP450 enzymes that could interact with lorlatinib (55). Patients may also experience mild changes in mental status. Such effects reported in the phase I–II studies were generally mild and improved or resolved upon dose interruptions or reductions. There appears to be a wide range of CNS side effects and includes changes in cognitive function (i.e., memory impairment, confusion, disturbances in attention), mood (i.e., irritability, anxiety, depression, flat affect, euphoria/mania), and speech (i.e., slowed speech, difficulty in word finding). Out of 117 patients who had CNS effects, 24 (20.5%) required one or more than one dose level of modification (such as temporary interruption and or dose reduction) with 15/24 (62.5%) of these patients having resolution of their CNS effects (56). The lorlatinib package insert recommends withholding the dose until grade 1 or lower and resuming at a reduced dose for grade 2 or 3 CNS effects and permanently discontinuing lorlatinib for grade 4 CNS events (57).
In general, NSCLC patients who harbor the ALK translocation are often young and highly functional. In those with occupations that require quick thinking and rapid processing (i.e., operation of machinery or stock exchange), lorlatinib may not be the optimal drug of choice. It is critical that not only patients but family members and care givers also be informed of the potential CNS effects of lorlatinib at the start of therapy and to be educated to notify their treating physicians as soon as such events were to occur.
Ongoing studies, combo strategies
Lorlatinib is in phase III testing to investigate whether first-line treatment with lorlatinib can further improve clinical outcomes for patients with metastatic ALK-rearranged NSCLC compared with first-line crizotinib treatment (ClinicalTrials.gov, NCT03052608, CROWN study). Given high CNS penetration of lorlatinib when compared to other ALK inhibitors, it would be important to document outcomes such as intracranial response rates and time to progression (which are included in the study’s secondary objectives), in addition to the traditional primary outcome of PFS.
At the current time, there are no established treatment combination strategies that includes an ALK TKI. Important to note are the potential for developing ALK-nondominant mechanisms of resistance, which was actually documented in 12 out of 20 (60%) in the aforementioned study by Yoda et al. Consideration of combination treatment with an ALK inhibitor with other agents that bypass different pathways such as MET, EGFR, KIT or SRC may be a valuable approach in those with ALK-nondominant mechanisms of resistance. For example, ALK inhibition with mitogen-activated protein kinase inhibition in mouse models have shown to prevent resistance and increase response to ALK inhibition (58) and this approach is being evaluated in clinical trials (NCT03202940: alectinib and cobimetinib, NCT03087448: ceritinib and trametinib).
Although immunotherapy has changed the landscape of NSCLC treatment, there are data to show that using single agent immune checkpoint inhibitors in those patients with sensitizing ALK-rearrangements may not be as efficacious (59) with the potential for increased toxicity when combined with an ALK TKI; indeed, enrollment for CheckMate-370, a study combining crizotinib and nivolumab as first-line treatment was discontinued due to significant hepatotoxicity (60). Therefore, careful selection of such combination or sequential approaches with immunotherapy must be examined.
Trials evaluating combination of ALK TKI and immune checkpoint inhibitors is shown in Table 2 (60-63).

Full table
The ALK Master Protocol/NRG Intergroup trial (NCT03737994) is an important study. In this trial, patients who have progressed on any ALK TKI will undergo a biopsy and a blood-based plasma test to look for acquired resistance mechanisms. Based on the results, the patients are assigned to an arm of different ALK inhibitors with or without combination of other targeted therapies. For example, those found to have ALK 1198F mutation or MET amplification will be assigned to receive crizotinib whereas those with compound mutations receive lorlatinib. Those with no ALK-resistance mutations are assigned to either lorlatinib, ceritinib, alectinib, brigatinib, ensartinib or pemetrexed with or without cisplatin/carboplatin.
In the adjuvant setting, the phase III ALCHEMIST screening trial (NCT02194738) is evaluating the utility of ALK TKIs in patients with early-stage ALK-rearranged NSCLC who have completed standard treatment such as chemotherapy after surgical resection. For the neoadjuvant setting, the Lung Cancer Mutation Consortium has proposed the PROMISE umbrella trial (LCMC4) to test for the presence of oncogenic drivers at diagnosis in patients with early stage lung cancer and to provide matched targeted therapies prior to surgical resection (64). Although there are currently five approved ALK inhibitors for NSCLC, there are currently no tumor agnostic approvals at this time. The My Pathway basket trial (NCT02091141) has a cohort evaluating the activity of alectinib in patients with ALK-mutated tumors (that are not NSCLC such as neuroblastoma and inflammatory myofibroblastic tumors).
Cost effectiveness
Studies evaluating the cost effectiveness of ALK TKIs are limited but generally supports the idea of utilizing second-generation ALK TKIs upfront. A US based study has shown that ceritinib is cost-effective when compared to chemotherapy and crizotinib in the treatment of treatment naïve ALK-positive metastatic NCSLC. Zhou et al., reported that upfront use of ceritinib was associated with total direct costs of $299,777 and 3.28 QALYs [from 4.61 life years gained (LYG)] over 20 years whereas upfront use of crizotinib and chemotherapy were associated with 2.73 and 2.41 QALYs, 3.92 and 3.53 LYG, and $263,172 and $228,184 total direct costs, respectively. The incremental cost per QALY gained was $66,064 for ceritinib vs. crizotinib and $81,645 for ceritinib vs. chemotherapy. In the first 2 years after starting treatment, ceritinib dominated crizotinib by conferring greater health benefits at reduced total costs (65).
Prevention of brain metastases utilizing alectinib may also be cost-effective. Considering the incidence of brain metastases in each treatment arm of the ALEX trial and costs associated with brain metastases, Burudpakdee et al., estimated that over the 24-month follow-up period, treatment with alectinib rather than crizotinib, could save on average between $35,254 and $41,434 per patient in healthcare costs related to brain metastases (66). As 45.3% of patients treated with crizotinib developed intracranial metastases compared to 7.2% of patients treated with alectinib, the potential cost savings of preventing brain metastases in ALK+ NSCLC patients appears to be significant.
Conclusions
ALK inhibitors have robust efficacy in ALK-rearranged lung cancers. Second-generation ALK inhibitors, alectinib and brigatinib have demonstrated superior PFS and CNS activity compared to first-generation TKI, crizotinib, in treatment naïve patients and generally used initially in the first-line setting. However, responses are not always durable and a majority of patients will develop resistance. Molecular sequencing at time of resistance may assist in the sequential selection of an ALK inhibitor. The development of ALK resistance mutations, particularly G1202R was associated significantly with having an EML4-ALK variant 3. Lorlatinib has demonstrated efficacy in EML4-ALK variant 3 and has been shown to be effective after resistance to first and second-generation ALK TKIs. Additional research to evaluate mechanisms of acquired resistance will be crucial to the development of next generation ALK TKIs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Silvia Novello, Francesco Passiglia) for the series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-331
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-331). The series “Looking for Chimeras in NSCLC: Widen Therapeutic Options Targeting Oncogenic Fusions” was commissioned by the editorial office without any funding or sponsorship. SHIO serves as an unpaid editorial board member of Translational Lung Cancer Research from Jun 2018 to Jun 2020. BX has received honorarium from Astra Zeneca and advisory fees from Astra Zeneca, Roche/Genentech, and Regeneron Pharmaceuticals, outside the submitted work. MN has received advisory fees from AstraZeneca, Caris Life Sciences, Daiichi Sankyo, Takeda, Novartis, research support from Tempus and travel support from An Heart Therapeutics, outside the submitted work. VWZ has received honoraria from AstraZeneca, Biocept, Roche-Foundation Medicine, Roche/Genentech, Takeda, and has stock ownership in TP Therapeutics, outside the submitted work. SHIO has stock ownership and was on the scientific advisory board of Turning Point Therapeutics Inc (until Feb 28, 2019), and has received speaker honorarium from Merck, Roche/Genentech, Astra Zeneca, Takeda/ARIAD and Pfizer; has received advisory fees from Roche/Genentech, Astra Zeneca, Takeda/ARIAD, Pfizer, Foundation Medicine Inc, Spectrum, outside the submitted work. RAS has received honoraria from Astra-Zeneca, AMGEN, BMS, Boehringer Ingelheim, Lilly, Merck, Novartis, Pfizer, Roche, Taiho, Takeda, and Yuhan; and research funding from Astra-Zeneca and Boehringer Ingelheim, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Shaw AT, Solomon B. Targeting anaplastic lymphoma kinase in lung cancer. Clin Cancer Res 2011;17:2081-6. [Crossref] [PubMed]
- Pikor LA, Ramnarine VR, Lam S, et al. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013;82:179-89. [Crossref] [PubMed]
- Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. [Crossref] [PubMed]
- Ou SHI, Zhu VW, Nagasaka M. Catalog of 5’ Fusion Partners in ALK-positive NSCLC Circa 2020. JTO Clinical and Research Reports 2020;1:100015. [Crossref]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med 2018;379:2027-39. [Crossref] [PubMed]
- Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654-67. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol 2019;14:1233-43. [Crossref] [PubMed]
- Camidge R, Kim HR, Ahn M, et al. Brigatinib vs crizotinib in patients with ALK inhibitor-naive advanced ALK+ NSCLC: Updated results from the phase III ALTA-1L trial. Ann Oncol 2019;30:ix183-202. [Crossref]
- Horn L, Infante JR, Reckamp KL, et al. Ensartinib (X-396) in ALK-positive non-small cell lung cancer: results from a first-in-human phase I/II, multicenter study. Clin Cancer Res 2018;24:2771-9. [Crossref] [PubMed]
- Mok TS, Kim D, Wu Y, et al. Overall survival (OS) for first-line crizotinib versus chemotherapy in ALK+ lung cancer: updated results from PROFILE 1014. Ann Oncol 2017;28:v605-49. [Crossref]
- Cho BC, Kim DW, Bearz A, et al. ASCEND-8: A randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with anaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol 2017;12:1357-67. [Crossref] [PubMed]
- Peters S, Mok T, Gadgeel SM, et al. Updated overall survival (OS) and safety data from the randomized, phase III ALEX study of alectinib (ALC) versus crizotinib (CRZ) in untreated advanced ALK+ NSCLC. J Clin Oncol 2020;38:abstr 9518.
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Zhou C, Kim SW, Reungwetwattana T, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med 2019;7:437-46. [Crossref] [PubMed]
- Gadgeel SM, Mok T, Peters S, et al. Phase II/III Blood First Assay Screening Trial (BFAST) in patients (pts) with treatment-naïve NSCLC: initial results from the ALK+ cohort. Ann Oncol 2019;30:v851-934. [Crossref]
- Lin JJ, Zhu VW, Schoenfeld AJ, et al. Brigatinib in patients with alectinib-refractory ALK-positive NSCLC. J Thorac Oncol 2018;13:1530-8. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Syed YY. Lorlatinib: first global approval. Drugs 2019;79:93-8. [Crossref] [PubMed]
- Horn L, Leal TA, Oxnard G, et al. OA03.08 Activity of ensartinib after second generation anaplastic lymphoma kinase (ALK) tyrosine kinase inhibitors (TKI): topic: medical oncology. J Thorac Oncol 2017;12:S1556. [Crossref]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol 2018;36:1199-206. [Crossref] [PubMed]
- Shaw AT, Solomon BJ, Besse B, et al. ALK resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol 2019;37:1370-9. [Crossref] [PubMed]
- Horn L, Whisenant JG, Wakelee H, et al. Monitoring therapeutic response and resistance: analysis of circulating tumor DNA in patients with ALK+ lung cancer. J Thorac Oncol 2019;14:1901-11. [Crossref] [PubMed]
- Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214-22. [Crossref] [PubMed]
- Califano R, Hochmair MJ, Gridelli C, et al. Brigatinib (BRG) vs crizotinib (CRZ) in the phase 3 ALTA-1L trial. Ann Oncol 2019;30:ii38-68. [Crossref]
- ESMO. Clinical Practice Living Guidelines – Metastatic Non-Small-Cell Lung Cancer. Available online: (Accessed 1 March, 2020).https://www.esmo.org/guidelines/lung-and-chest-tumours/metastatic-non-small-cell-lung-cancer
- Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. [Crossref] [PubMed]
- Gainor JF, Tan DS, De Pas T, et al. Progression-free and overall survival in alk-positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin Cancer Res 2015;21:2745-52. [Crossref] [PubMed]
- Chiari R, Metro G, Iacono D, et al. Clinical impact of sequential treatment with ALK-TKIs in patients with advanced ALK-positive non-small cell lung cancer: results of a multicenter analysis. Lung Cancer 2015;90:255-60. [Crossref] [PubMed]
- Ito K, Hataji O, Kobayashi H, et al. Sequential therapy with crizotinib and alectinib in ALK-rearranged non-small cell lung cancer-a multicenter retrospective study. J Thorac Oncol 2017;12:390-6. [Crossref] [PubMed]
- Iams WT, Lovly CM. Anaplastic lymphoma kinase as a therapeutic target in non-small cell lung cancer. Cancer J 2015;21:378-82. [Crossref] [PubMed]
- NCCN. Guidelines for Non-Small Cell Lung Cancer. Available online: (Accessed 1 March, 2020).https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Lin JJ, Schoenfeld AJ, Zhu VW, et al. Efficacy of platinum/pemetrexed combination chemotherapy in ALK-positive NSCLC refractory to second-generation ALK inhibitors. J Thorac Oncol 2020;15:258-65. [Crossref] [PubMed]
- Hida T, Seto T, Horinouchi H, et al. Phase II study of ceritinib in alectinib-pretreated patients with anaplastic lymphoma kinase-rearranged metastatic non-small-cell lung cancer in Japan: ASCEND-9. Cancer Sci 2018;109:2863-72. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Sasaki T, Rodig SJ, Chirieac LR, et al. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer 2010;46:1773-80. [Crossref] [PubMed]
- Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol 2009;27:4232-5. [Crossref] [PubMed]
- Yoshida T, Oya Y, Tanaka K, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non-small-cell lung cancer. J Clin Oncol 2016;34:3383-9. [Crossref] [PubMed]
- Mok T, Peters S, Camidge DR, et al. MA 07.01 Patients with ALK IHC-positive/FISH-negative NSCLC benefit from ALK TKI treatment: response data from the global ALEX trial. J Thorac Oncol 2017;12:S1826. [Crossref]
- Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Ou SH, Weitz M, Jalas JR, et al. Alectinib induced CNS radiation necrosis in an ALK+NSCLC patient with a remote (7 years) history of brain radiation. Lung Cancer 2016;96:15-8. [Crossref] [PubMed]
- Ou SH, Klempner SJ, Azada MC, et al. Radiation necrosis presenting as pseudoprogression (PsP) during alectinib treatment of previously radiated brain metastases in ALK-positive NSCLC: implications for disease assessment and management. Lung Cancer 2015;88:355-9. [Crossref] [PubMed]
- Zhu VW, Nagasaka M, Kubota T, et al. Symptomatic CNS radiation necrosis requiring neurosurgical resection during treatment with lorlatinib in ALK-rearranged NSCLC: a report of two cases. Lung Cancer (Auckl) 2020;11:13-8. [Crossref] [PubMed]
- Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol 2018;13:1248-68. [Crossref] [PubMed]
- Yoda S, Lin JJ, Lawrence MS, et al. Sequential ALK inhibitors can select for lorlatinib-resistant compound ALK mutations in ALK-positive lung cancer. Cancer Discov 2018;8:714-29. [Crossref] [PubMed]
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Neuvonen PJ, Niemi M, Backman JT. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin Pharmacol Ther 2006;80:565-81. [Crossref] [PubMed]
- Bauer TM, Felip E, Solomon BJ, et al. Clinical management of adverse events associated with lorlatinib. Oncologist 2019;24:1103-10. [Crossref] [PubMed]
- Highlights of prescribing information: Lorbrena (lorlatinib). Available online: (Accessed 1 April, 2020).https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210868s000lbl.pdf
- Hrustanovic G, Olivas V, Pazarentzos E, et al. RAS-MAPK dependence underlies a rational polytherapy strategy in EML4-ALK-positive lung cancer. Nat Med 2015;21:1038-47. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation - positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol 2018;13:682-8. [Crossref] [PubMed]
- Felip E, de Braud FG, Maur M, et al. Ceritinib plus nivolumab in patients with advanced ALK-rearranged non-small cell lung cancer: results of an open-label, multicenter, phase 1B study. J Thorac Oncol 2020;15:392-403. [Crossref] [PubMed]
- Shaw AT, Lee S-H, Ramalingam SS, et al. Avelumab (anti-PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: phase 1b results from JAVELIN Lung 101. J Thorac Oncol 2018;36:9008.
- Kim D-W, Gadgeel SM, Gettinger SN, et al. Safety and clinical activity results from a phase Ib study of alectinib plus atezolizumab in ALK+ advanced NSCLC (aNSCLC). J Thorac Oncol 2018;36:9009.
- Bauer TM, Papadopoulos KP, Autio KA, et al. A first-in-human study of pegylated recombinant human IL-10 (AM0010), daily administered for four months in selected advanced solid tumors. ASCO Meeting Abstracts 2014;32:TPS3126.
- Zhou ZY, Mutebi A, Han S, et al. Cost-effectiveness of ceritinib in previously untreated anaplastic lymphoma kinase-positive metastatic non-small cell lung cancer in the United States. J Med Econ 2018;21:577-86. [Crossref] [PubMed]
- Burudpakdee C, Wong W, Seetasith A, et al. Economic impact of preventing brain metastases with alectinib in ALK-positive non-small cell lung cancer. Lung Cancer 2018;119:103-11. [Crossref] [PubMed]



