Dose-response relationship of stereotactic body radiotherapy for ultracentral tumor and comparison of efficacy with central tumor: a meta-analysis
Introduction
Lung cancer has long been the primary cause of cancer-related deaths (1), and surgical resection has been the standard curative modality for early-stage disease (2,3), Although external beam radiotherapy (EBRT) had previously been applied for locally advanced or metastatic disease, precise tumor targeting using updated technologies namely, stereotactic body radiotherapy (SBRT), enable curative treatment. Unlike conventional EBRT, SBRT delivers a very high dose precisely to the target in a relatively short period, and recent randomized trials have shown that outcomes post-SBRT were comparable to those following surgery (4,5).
The feasibility and efficacy of SBRT for treating peripheral tumors were previously demonstrated, but those for treating central tumors remain unclear. An early trial found that the risk of grade ≥3 toxicity was 11-fold higher when using SBRT to treat central tumors, defined as those within a 2 cm radius of the proximal bronchial tree (PBT), than treating peripheral tumors (6). However, central tumors are commonly inoperable or require more extensive surgery than do peripheral tumors; hence, many researchers have continued administering SBRT to central tumors using more protracted regimens. With the downside of having a moderate risk level of complications (the grade ≥3 complication rate is ~9%), the oncologic outcomes of patients treated with SBRT for central tumors were comparable to those treated for peripheral tumors (7,8).
While careful administration of SBRT to central tumors has been performed, an independent concept of “ultracentral” (UC) tumors was introduced; this generally refers to tumors that abut the PBT (9). When SBRT is applied to such tumors, the target volume must cover the PBT, rendering its irradiation with the full treatment dose inevitable. Corradetti et al. (10). described a patient who developed fatal central airway necrosis owing to UC tumor irradiation and, therefore, warned of the risk of such a treatment. However, UC tumors are more intractable and have fewer curative options than do central tumors. In early studies, some investigators reported serious toxicity rates of over 20% (11,12), while other studies reported more favorable results (4,13).
Several researchers eagerly reported their clinical experiences recently, and their data were more encouraging than in the past. A suitable SBRT dose information for UC tumors has been longed by clinicians. At the same time, whether UC tumors have different outcomes post-SBRT from central tumors is controversial (13-17). Hence, the present meta-analysis was performed to assess the dose-response relationship and feasibility of SBRT for UC tumors and to compare outcomes between UC and central tumors.
We present the following article in accordance with the PRISMA reporting Guideline (available at http://dx.doi.org/10.21037/tlcr-20-503).
Methods
The present meta-analysis and systematic review were performed to address the following clinical (PICO) questions: “(I) Does SBRT for UC tumors have feasibility and dose-response relationship? (II) Are UC tumors a distinct clinical subset of central tumors for purposes of considering SBRT application?” Databases including MEDLINE and EMBASE were searched for records available up to March 1, 2020. We used the following search terms: (ultracentral OR “ultra central” OR “ultra-central”) and lung and (“radiation therapy” or “radiotherapy”). Reference lists from the searched articles were used to locate additional publications. No language or time restrictions were applied. Unpublished literature was considered if it fully satisfied the inclusion criteria.
Inclusion criteria
Eligible studies included in the present meta-analysis met all of the following criteria: (I) clinical trials; (II) inclusion of ≥5 patients with UC tumors who underwent SBRT; (III) definition of UC tumor must include “abutting the PBT” (e.g., tumors described as “<1 cm from the PBT” were not included, as they could encompass central tumors); (IV) SBRT was delivered at either >4 Gy per fraction or in ≤10 fractions; (V) SBRT was not performed in re-irradiation setting and 5) at least one of the primary endpoints was reported. The primary endpoints were rates of local control (LC) and overall survival (OS), while secondary endpoints were of grade ≥3 or grade 5 complications. Initial screening was performed using citations and titles to filter out duplicate studies, reviews, editorials, letters, and in vivo or in vitro studies. Abstracts were reviewed to exclude studies with irrelevant subjects or formats. Full-text reviews were then performed to identify studies that fulfilled all the inclusion criteria. Multiple studies from the same institutions were sorted using the following criteria—prioritized in numerical order: (I) studies with the largest number of patients with UC; (II) published articles were preferred over conference abstracts. We included multiple studies from the same institution if they had no overlapping patients or if the overlap was negligible. Screening for these inclusion criteria was performed by two independent researchers, and final inclusion was decided upon mutual consent.
Data collection
Data collection from the included studies was performed using a pre-designed standardized form to evaluate (I) background information including authors, affiliations, study type, and number of patients; (II) clinical information including T stage, proportion of squamous histology cases, target volume, proportion of metastasis or recurrence cases, SBRT dose, and definition of UC tumor; (III) outcomes of interest including LC, OS, and complication of grade ≥3. LC and OS rates were estimated from descriptive graphs, considering follow-up periods, in the absence of numeral data. The prescribed SBRT doses were converted to the biologically equivalent dose (BED) using an α/β ratio of 10 and equivalent dose in 2 Gy fractions (EQD2) using α/β ratios of 3 and 10. An α/β ratio of 10 represented aggressive biologic behavior in the early responding tissues (i.e., the tumors), while an α/β ratio of 3 represented that in late-responding tissues and was commonly used to estimate complication risks (18). The data collection process was performed by two independent researchers, and disagreements were resolved by conducting an additional literature review and mutual discussion.
Quality assessment
As most of the included studies were retrospective, the Newcastle-Ottawa scale (19) was used for quality assessment. Studies with scores of 7–9 and 4–6 were considered to be of high and medium qualities, respectively.
Statistical analysis
Pooled analyses were performed for all primary and secondary endpoints. The selection of the effects model depended on the nature of the included studies and their data, rather than on the calculated heterogeneity (20). A random-effects model was used considering the inevitable heterogeneity of the patients’ characteristics and treatment details (21). Heterogeneity among the studies was assessed using Cochran Q test and I2 statistics; (22,23). Significant heterogeneity was considered present when P<0.1 and I2≥50%; I2 values of 25%, 50%, and 75% corresponded to low, moderate, and high degrees of heterogeneity, respectively. Pooled analyses of 1- and 2-year LC and OS rates were performed for controlled studies, whereas only analyses of 1-year LC and OS rates were performed for all studies because the follow-up durations were short in single-arm UC case series. Subgroup comparisons were performed using Q tests on the basis of analysis of variance, and P values <0.05 indicated significant differences among the subgroups. Meta-regression was performed to quantitatively assess the relationship between the endpoints and BED10Gy, and P values <0.05 represented significant correlations. Publication biases were evaluated via the visual inspection of funnel plots, quantitative results of the Egger’s test, and analyzing Rosenthal’s fail-safe number (24,25). If funnel plot inspection showed asymmetric distributions and the 2-tailed P value of the Egger’s test was <0.1, then the fail-safe number was calculated; if the possibility arose that studies similar to that number may have been missed, Duval and Tweedie’s trim and fill method (26) was used to determine the corrected relevant values. All statistical analyses were conducted using the Comprehensive Meta-Analysis software version 3 (Biostat, Inc., Englewood, NJ).
Results
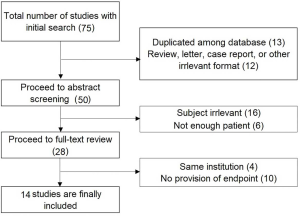
Among the 72 studies that were initially searched, 14 studies including 892 patients (411 and 481 with UC and central tumors, respectively), were finally included (9,11-17,27-32). The study inclusion process is described in Figure S1. Eight of the studies were controlled trials that included patients with both UC and other central tumors, whereas six were single-arm observational studies of patients with UC. Two studies from Georgetown University were included in the final list (28,29), as the authors unanimously agreed that the number of overlapping patients was small enough not to yield a significant bias whereas including the data would enrich the pooled analyses. We also included our older data that were reported in a previous publication and subsequently updated (30,33). All the included studies were described in full-text articles. Six single-arm observational studies (11,27-30,32) were categorized as having medium quality according to the Newcastle-Ottawa scale, and 8 controlled studies (9,13-17) were considered high-quality.
The proportions of T1 tumors ranged from 2% to 76% with a median of 51.8%. The median PTV ranged from 23.2 to 111.3 cm3, with a median of 68.5 cm3. The median prescribed dose ranged from 59.5 to 132 BEDGy10, with a median of 100 BEDGy10. Basic information about the included studies is summarized in Table 1, while clinical information is shown in Table 2.
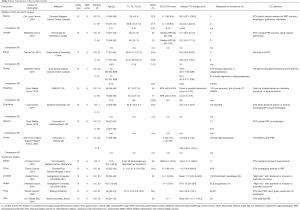
Full table
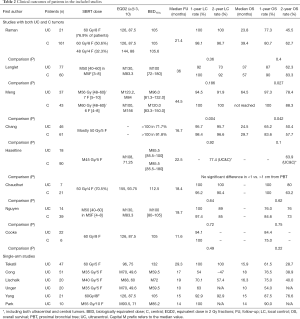
Full table
Comparison of UC and other central tumors
In pooled analyses of the controlled studies, the 1-year LC rates were 93.9% (95% CI: 95.6–98.9%) and 97.8% (95% CI: 95.6–98.9%) in the UC and central tumor groups, respectively (P=0.023), while the corresponding 2-year LC rates were 90.4% (95% CI: 77.8–96.2%) and 93.7% (95% CI: 88.3–96.7%), respectively (P=0.459). Moreover, the corresponding 1-year OS rates were 82.2% (95% CI: 71.7–89.7%) and 85.4% (95% CI: 78.9–90.1%), respectively (P=0.556), while the 2-year OS rates were 66.4% (95% CI: 51.4–78.7%) and 71.9% (95% CI: 61.0–80.8%), respectively (P=0.522). The pooled grade ≥3 complication rates in the UC and central tumor groups were 9.0% (95% CI: 5.0–15.9%) vs. 4.4% (95% CI: 2.8–6.9%) (P=0.06), while the grade 5 complication rates were 5.7% (95% CI: 2.6–11.9%) vs. 2.3% (95% CI: 1.1–4.6%) (P=0.087). The results of the pooled analyses are summarized in Table 3 and are also shown as forest plots in Figure 1.
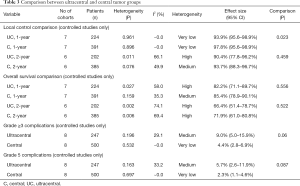
Full table
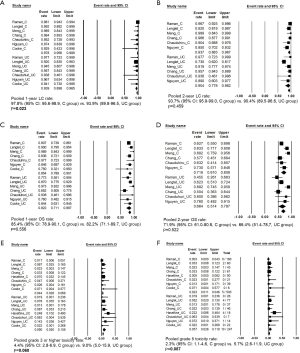
Pooled analyses among all UC cohort
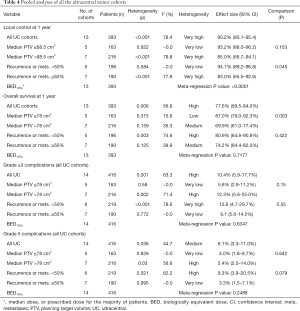
In a pooled analyses of all UC cohorts from controlled and single-arm studies, the 1-year LC and OS rates were 90.2% (95% CI: 80.1–95.4%) and 77.6% (95% CI: 69.5–84.0%), respectively; moreover, grades ≥3 and 5 complication rates were 10.4% (95% CI: 5.9–17.7%) and 6.1% (95% CI: 3.3–11.0%), respectively. Subgroup comparisons were performed according to the percent of patients in a study with recurrence or metastases, and median PTV volume. On subgroup comparisons, the 1-year LC rates were 94.1% (95% CI: 89.2–96.8%) and 83.0% (95% CI: 65.5–92.6%) in the subgroups representing recurrence or metastases incidences of <50% and ≥50%, respectively (P=0.045). The pooled 1-year OS rates were 87.0% (95% CI: 79.0–92.3%) and 69.9% (95% CI: 61.0–77.4%) in the subgroups representing median PTVs of ≤78 and >78 cm3, respectively (P=0.003). Above results are summarized in Table 4.
Full table
Dose-response for LC among all UC cohort (meta-regression analyses)
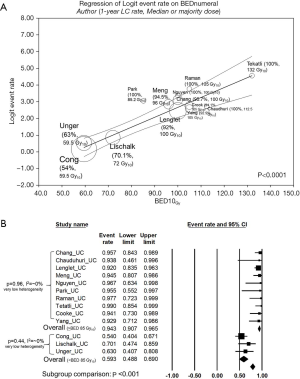
The median BED10Gy of the studies was significantly correlated with the 1-year LC rate (P<0.0001, Figure 2A). With the threshold of 85 Gy10, commonly prescribed as 55 Gy in 10 fractions or 45 Gy in 5 fractions, 1-year LC rates were significantly different (94.4% vs. 59.3%, P<0.001) with very low heterogeneities in both subgroups (I2=~0% in both); the result is shown in Figure 2B in detail. Meta-regression results were not statistically significant between the median BED10 Gy and 1-year OS, grade ≥3 complications, and grade 5 complications. The results of meta-regression analyses are shown in Table 4. Forest plots and scatterplots of meta-regression were shown in Figure S2.

Qualitative analysis of fatal complications
As fatal complications are the most important concern when applying SBRT for UC tumors, we qualitatively analyzed the reported toxicities in addition to the pooled analyses. Data regarding fatal complications were available for all the included studies involving 892 patients (411 and 481 with UC and central tumors); fatal complications were reported in 28 patients. Among them, 24 had UC tumors while 4 had other central tumors. The fatal complications among patients with UC tumors were hemorrhage (9, 37.5%), pneumonia or respiratory failure (9, 37.5%), bronchial stenosis or fistula (3, 12.5%), and cardiac toxicity (3, 12.5%). Fatal complications among patients with central tumors included pneumonitis (3, 75%) and myocardial infarction (1, 25%). The reported complication rates and their suggested risk factors are shown in Table 5.

Full table
Publication bias assessment
Egger’s test showed that possible publication bias was present for the 1-year LC rate (P<0.001), 2-year LC rate (P=0.005), 1-year OS rate (P=0.051), grade ≥3 complication rate (P=0.003), and grade 5 complication rate (P<0.001). The fail-safe numbers (e.g., the numbers of unpublished or unfound studies enough to statistically nullify observed effects) to prevent publication bias were 889, 719, 877, 1,348, and 1,294, respectively. Since it is unreasonable to assume that studies with these numbers were missing from our literature search, the originally observed effect sizes rather than adjusted values were shown as results.
Discussion
Tumors abutting the PBT are generally either inoperable or require extensive surgery such as pneumonectomy (11,34). Therefore, the feasibility of SBRT, which can provide a curative opportunity, is of great clinical significance. The application of SBRT for UC tumors had been considered impossible until just a few years ago, and the feasibility of SBRT was gradually acknowledged by pioneering researchers (35). We previously performed a meta-analysis of the literature published until April 2018 (36). Surprisingly, our current study (merely 2 years later) included 3 times as many full-text publications as did the previous meta-analysis; this reflects the rigorous academic interest in the subject. In addition to the merits of including more research articles, the present study confers comparative results between UC and central tumor and dose-response for LC and suggestion of dose prescription of SBRT for UC tumors.
Regarding the primary outcomes of the study, the pooled OS rates at 1 and 2 years were not different between the UC and central tumor groups. Although the 1-year LC rates for the UC and central tumor groups were significantly different (93.9% vs. 97.8%, P=0.023), the clinical impact of this difference might be moderate considering that the 2-year LC rates were not significantly different and the 1-year LC rates in both groups were very high. The narrow range of 1-year LC rates reported as well as the very low heterogeneity between the studies might also have produced the statistical difference. Taken together, oncologic outcomes after SBRT for UC and central tumors are thought to be very similar to outcomes in clinical practice.
In the pooled analyses of all UC tumor cohorts, the 1-year LC and OS rates were favorable (90.2% and 77.6%, respectively), demonstrating the efficacy of SBRT. Subgroup comparisons revealed that a higher proportion of recurrence or metastasis influenced the LC rate (P=0.045), while the OS rate was largely affected by tumor size (P=0.003); these results were expected when considering current knowledge of tumor biology (37-39). The dose-response relationship for LC was significant in the meta-regression (P<0.0001, Figure 2A). However, an overwhelming majority of studies used SBRT doses near or mildly higher from 100 Gy10, reflecting that most oncologists tended to prescribe higher doses than those recommended by Onishi et al. (40). and were concerned for possible toxicities simultaneously. The prescription dose of 59.5–72 Gy10 was shown to be suboptimal (27-29) as it only showed a pooled 1-year LC rate of 59.3%. With the threshold of 85 Gy10, all studies with higher prescription doses reported a 1-year LC rate of over 90%, and the pooled rate was favorable at 94.3% with very low heterogeneity (Figure 2B). Therefore, we suggest the application of SBRT with a dose of at least 85 Gy10, which can be prescribed as either 55 Gy in 10 fractions (30) or 45 Gy in 5 fractions (12). Dose over 100 Gy10 can be prescribed with an expectation of a dose-response relationship; however, risk factors should be monitored as SBRT for UC tumors have a higher risk of complications than that for central tumors. Feasibility and additional efficacy regarding long-term LC of dose escalation should be evaluated in future studies.
The main reason UC tumors began to be treated independently of central tumors was that the former were thought to be more vulnerable to serious toxicities. Haseltine et al. (12). and Tekatli et al. (11), whose studies were performed relatively early, reported overwhelmingly high toxicity rates (grade ≥3 complications rates in these studies were 24.8% and 38%, respectively), thereby causing reluctance regarding the feasibility of SBRT. On the other hand, studies by Lenglet et al. (41), Raman et al. (13), and Chang et al. (15). found that the differences in serious toxicities between central and UC tumors were not significant. Given the inevitable full-dose irradiation to PBT when treating UC tumors, and results of higher grade ≥3 complications rates (9% vs. 4.4%, P=0.06) and grade 5 complication (5.7% vs. 2.3%, P=0.087) with UC than with central tumors post-SBRT (with borderline significances), it seems that irradiating UC tumors produces a greater susceptibility to serious toxicities than does irradiating central tumors.
The pooled grade ≥3 complication rate was 10.4%, which was much lower than that revealed in the previous meta-analysis (23.2%) (36). The pooled complication rates in previous meta-analyses were largely affected by those of Tekatli et al. (11). and Haseltine et al. (12), which reported relatively high rates of 24.8% and 38%. However, in Tekatli et al.’s study (11), 60% of the tumors were >5 cm in diameter and 32% were >7 cm; these sizes were much larger than those of tumors commonly indicated for SBRT. In Haseltine et al.’s study (12), it was not clear that SBRT was the main cause of complications because some patients also received bevacizumab, exposure to which is a known risk factor for serious hemorrhage when treating central lung cancer (42). In fact, the authors also suggested that bevacizumab might have contributed to hemorrhagic complications, and also noted that gram-negative bacterial pneumonia (another serious complication that arose) is generally not caused by non-invasive treatments such as SBRT. Contrarily, more recent trials including those by Lenglet et al. (14), Meng et al. (17), Raman et al. (13), and Chang et al. (15). which might avoid such risks previously suggested, found much more acceptable rates of toxicity (0% to ~8%) although all patients in these studies were prescribed more than 100 Gy10.
Grade 5 complication is the most significant factor determining the application of SBRT for UC tumors reluctant. Of note, most researchers reported factors that may have significantly influenced fatal toxicity. Tekatli et al. (11). reported 10 patients among 47 with UC tumors who experienced fatal toxicity, including 7 with hemorrhages. Anticoagulant use, squamous histology, excessive irradiation dose (DMax >123%), and endobronchial involvement were the presumed causes of such toxicities. Haseltine et al. (12). reported fatal toxicities in 4 of their 18 patients with UC tumors (22.5%) and claimed that bevacizumab exposure might have caused fatal hemorrhagic toxicities. Studies by Chang et al. (15), Meng et al. (17), Lenglet et al. (14), and Unger et al. (29). revealed much lower fatal toxicity rates (2–5%) than did the previous 2 studies, suggesting that underlying lung diseases such as interstitial lung disease and idiopathic pulmonary fibrosis might have been associated with fatal respiratory toxicities. Although none of the individual studies reported statistically significant differences in serious toxicities between patients with UC and central tumors (9,12,13,15,41), considering the small patient number of individual studies and borderline significance found in our subgroup comparisons, we support the notion that applying SBRT for UC tumors carries a greater risk of fatal toxicities than does applying it for central tumors. Hence, all risk factors suggested by previous investigators should be taken into consideration before treatment.
Limitations of the present meta-analysis include the non-randomized and retrospective design, and the clinical heterogeneity of the patients. Meta-analyses of observational studies are controversial because innate heterogeneity among studies might affect the pooled estimates (43). SBRT for UC tumors was contraindicated until recently when the indication was updated following pioneering research. As UC tumors are commonly inoperable or else require extensive surgeries, assessment of other curative modalities such as SBRT is crucial. In such situations, meta-analyses of observational studies can be one of the few options that provide helpful information for clinical practice (43). Short follow-up periods are another limitation in recent studies; we did not perform pooled analyses of the 2-year outcome rates in all UC cohorts because the available data were limited and follow-up periods in UC case series were too short. It should be considered that reporting of late toxicity events depends on follow-up and that risks of fatal toxicities may be higher. The heterogeneity of definitions for UC tumors is another drawback that ought to be resolved. The assessment of the feasibility or efficacy of treatment for UC tumors might be difficult if the definition of the target disease is unclear. We suggest that future studies use agreeable definitions and terminology regarding UC tumors.
Conclusions
The oncologic outcomes of SBRT for patients with UC and central tumors were comparable, although patients treated for UC tumors are more prone to serious toxicities. Nevertheless, SBRT for UC tumors is feasible considering the moderate rate of toxicities and the clinical need for a non-invasive curative modality. Considering the dose-response relationship, a dose of at least 85 Gy10 is recommended to be prescribed, and doses near or moderately higher than 100 Gy10 can be considered with cautious monitoring for risk factors of complications. Studies with longer follow-up which enable assessments of higher dose for sustained LC are warranted. The identified causes of fatal toxicities should be avoided in clinical practice as much as possible.
Acknowledgments
Funding: This study is supported by National research fund of Korea (NRF-2018R1D1A1B07046998). The research grant supported only methodological aspects including statistical analysis and linguistic correction, and did not affect major contents including the results and conclusions.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-503
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-503). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- National Cancer Comprehensive Network (NCCN). Principles of radiation therapy (NSCL-C), Non-small cell lung cancer. NCCN guidelines version 4.2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax 2015;70 Suppl 2:ii1-54. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Hiley C, Salem A, Batchelor T, et al. Great debate: surgery versus stereotactic radiotherapy for early-stage non-small cell lung cancer. Thorax 2020;75:198-9. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Senthi S, Haasbeek CJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for central lung tumours: a systematic review. Radiother Oncol 2013;106:276-82. [Crossref] [PubMed]
- Yu T, Shin I, Yoon WS, et al. Stereotactic body radiotherapy for centrally located primary non-small cell lung cancer: a meta-analysis. Clin Lung Cancer 2019;20:e452-62. [Crossref] [PubMed]
- Chaudhuri AA, Tang C, Binkley MS, et al. Stereotactic ablative radiotherapy (SABR) for treatment of central and ultra-central lung tumors. Lung Cancer 2015;89:50-6. [Crossref] [PubMed]
- Corradetti MN, Haas AR, Rengan R. Central-airway necrosis after stereotactic body-radiation therapy. N Engl J Med 2012;366:2327-9. [Crossref] [PubMed]
- Tekatli H, Haasbeek N, Dahele M, et al. Outcomes of hypofractionated high-dose radiotherapy in poor-risk patients with “ultracentral” non–small cell lung cancer. J Thorac Oncol 2016;11:1081-9. [Crossref] [PubMed]
- Haseltine JM, Rimner A, Gelblum DY, et al. Fatal complications after stereotactic body radiation therapy for central lung tumors abutting the proximal bronchial tree. Pract Radiat Oncol 2016;6:e27-33. [Crossref] [PubMed]
- Raman S, Yau V, Pineda S, et al. Ultracentral Tumors Treated With Stereotactic Body Radiotherapy: Single-Institution Experience. Clin Lung Cancer 2018;19:e803-10. [Crossref] [PubMed]
- Lenglet A, Campeau MP, Mathieu D, et al. Risk-adapted stereotactic ablative radiotherapy for central and ultra-central lung tumours. Radiother Oncol 2019;134:178-84. [Crossref] [PubMed]
- Chang JH, Poon I, Erler D, et al. The safety and effectiveness of stereotactic body radiotherapy for central versus ultracentral lung tumors. Radiother Oncol 2018;129:277-83. [Crossref] [PubMed]
- Nguyen KNB, Hause DJ, Novak J, et al. Tumor control and toxicity after SBRT for ultracentral, central, and paramediastinal lung tumors. Pract Radiat Oncol 2019;9:e196-202. [Crossref] [PubMed]
- Meng MB, Wang HH, Zaorsky NG, et al. Risk-adapted stereotactic body radiation therapy for central and ultra-central early-stage inoperable non-small cell lung cancer. Cancer Sci 2019;110:3553. [Crossref] [PubMed]
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011.
- Borenstein M, Hedges LV, Higgins JP, et al. Introduction to meta-analysis. Hoboken: John Wiley & Sons, 2011.
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007;28:105-14. [Crossref] [PubMed]
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101-29. [Crossref]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Rosenthal R. Combining results of independent studies. Psychological Bulletin 1978;85:185-93. [Crossref]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [Crossref] [PubMed]
- Cong Y, Sun B, Wang J, et al. Outcomes and toxicity of stereotactic body radiation therapy for advanced stage ultra-central non-small cell lung cancer. Thorac Cancer 2019;10:1567-75. [Crossref] [PubMed]
- Lischalk JW, Malik RM, Collins SP, et al. Stereotactic body radiotherapy (SBRT) for high-risk central pulmonary metastases. Radiat Oncol 2016;11:28. [Crossref] [PubMed]
- Unger K, Ju A, Oermann E, et al. CyberKnife for hilar lung tumors: report of clinical response and toxicity. J Hematol Oncol 2010;3:39. [Crossref] [PubMed]
- Park S, Kim Y, Yoon WS, et al. A preliminary experience of moderate-intensity stereotactic body radiation therapy for ultra-central lung tumor. Int J Radiat Biol 2019;95:1287-94. [Crossref] [PubMed]
- Cooke R, Camilleri P, Chu KY, et al. Stereotactic body radiotherapy for moderately central and ultra-central oligometastatic disease: Initial outcomes. Tech Innov Patient Support Radiat Oncol 2020;13:24-30. [Crossref] [PubMed]
- Yang D, Cui J, Zhao J, et al. Stereotactic ablative radiotherapy of 60 Gy in eight fractions is safe for ultracentral non-small cell lung cancer. Thorac Cancer 2020;11:754-61. [Crossref] [PubMed]
- Stereotactic body radiotherapy for centrally located lung tumor. Annual conference of Korean Lung Cancer Association; 2019, Seoul, Korea.
- Gómez-Caro A, Garcia S, Reguart N, et al. Determining the appropriate sleeve lobectomy versus pneumonectomy ratio in central non-small cell lung cancer patients: an audit of an aggressive policy of pneumonectomy avoidance. Eur J Cardiothorac Surg 2011;39:352-9. [Crossref] [PubMed]
- Chen H, Laba JM, Zayed S, et al. Safety and effectiveness of stereotactic ablative radiotherapy for ultra-central lung lesions: a systematic review. J Thorac Oncol 2019;14:1332-42. [Crossref] [PubMed]
- Rim CH, Kim Y, Kim CY, et al. Is stereotactic body radiotherapy for ultra-central lung tumor a feasible option? A systemic review and meta-analysis. Int J Radiat Biol 2019;95:329-37. [Crossref] [PubMed]
- Milano MT, Kong FM, Movsas B. Stereotactic body radiotherapy as salvage treatment for recurrence of non-small cell lung cancer after prior surgery or radiotherapy. Transl Lung Cancer Res 2019;8:78-87. [Crossref] [PubMed]
- Dunlap NE, Larner JM, Read PW, et al. Size matters: a comparison of T1 and T2 peripheral non–small-cell lung cancers treated with stereotactic body radiation therapy (SBRT). J Thorac Cardiovasc Surg 2010;140:583-9. [Crossref] [PubMed]
- Kim E, Song C, Kim MY, et al. Long-term outcomes after salvage radiotherapy for postoperative locoregionally recurrent non-small-cell lung cancer. Radiat Oncol J 2017;35:55-64. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- Lenglet A, Mathieu D, Campeau MP, et al. Risk-Adapted Lung SBRT for Central and Ultra-Central Tumors. Int J Radiat Oncol Biol Phys 2017;99:E473-4. [Crossref]
- Goto K, Endo M, Kusumoto M, et al. Bevacizumab for non-small-cell lung cancer: A nested case control study of risk factors for hemoptysis. Cancer Sci 2016;107:1837-42. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]