Expert consensus on image-guided radiofrequency ablation of pulmonary tumors—2015 edition
Introduction
Lung cancer is one of the most common malignant tumors worldwide. According to the GLOBOCAN 2012 published by the International Agency for Research on Cancer (IARC) under the World Health Organization (WHO), in 2012 there were about 1.80 million new lung cancer cases and 1.60 deaths (1). In China, data from ten tumor registries showed that the morbidity and mortality of lung cancer have increased by 1.63% (1.30% in males and 2.34% in females, P<0.05) annually from 1988 to 2005 (2). The mortality rate of lung cancer has increased by 465% in China than 30 years ago, and about 600,000 people die of lung cancer each year (2,3).
While surgery remains the treatment of choice for early lung cancer, only 20-30% of lung cancer patients are feasible for surgical treatment in clinical settings. The proportions of elderly and middle-aged lung cancer patients continue to rise as the population ages. These patients often are accompanied by other conditions and therefore are not feasible or can not tolerate the conventional surgical resection. Thus, many new local treatment methods, such as minimally invasive tumor ablation, has been developed (Table 1) (4). Minimally invasive tumor ablation allows the maximal inactivation of tumor cells (with tumor as the center) and the normal tissues 0.5-1 cm away from the tumor with the maximal protection of normal lung tissues. As a minimally invasive, safe, conformal, simple, and repeatable technique, it has reliable effectiveness and enables rapid recovery, with few complications. Now it has become the fourth leading treatment for tumors. Currently, the common minimally invasive ablation techniques for lung tumors include radiofrequency ablation (RFA), cryoablation, and microwave ablation. However, only RFA has been listed in the US National Comprehensive Cancer Network (NCCN) clinical guidelines on non-small cell lung cancer (NSCLC).
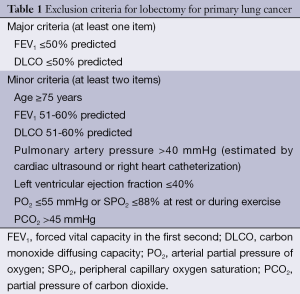
Full table
During the RFA, high-frequency alternating currents with a frequency of <30 MHz (normally 460-480 kHz) are used to make the ions in tumor tissues to develop rapid turbulence and mutual friction, transforming the radiofrequency (RF) energy into heat energy; as a result, the local temperature is escalated to 60-100 °C, which causes the tumor cells to develop coagulative necrosis. In 2000, Dupuy et al. (5) described the use of percutaneous RFA in treating three cases of lung tumor; since then, the clinical application of RFA for lung cancer has been widely reported (6-13). The effectiveness of RFA ablation depends on the transfer of heat produced by local RFA and thermal convection between the blood circulation and extracellular fluid (14). However, the lungs have the following features: (I) with rich blood supply; (II) as an air-bearing organ; and (III) with respiratory movement. Therefore, RFA may be less effective and/or accompanied with high local relapse rate due to heat sinking and high impedance (mean impedance value: 509±197 Ω) (7,15,16). Complications (e.g., pneumothorax) can be common. The efficacy evaluation may be different from other solid organs; for example, special attention may be paid to the ground-glass opacity (GGO) around the tumor after the ablation. Therefore, although RFA is a minimally invasive technique for lung cancer, it still has potential risks and may be associated with life-threatening complications. During the period from October to November 2014, under the leadership of Chinese Society of Thoracic and Cardiovascular Surgery Lung Cancer Study Group, Dr. Bao-Dong Liu from the Department of Thoracic Surgery, Xuanwu Hospital of Capital University of Medical sciences, drafted the expert concensus document, which was reviewed by Dr. Xiu-Yi Zhi and then asked for further consultations from experts in this field via meetings and emails. Finally, this Expert Consensus on Image-guided Radiofrequency Ablation of Pulmonary Tumors was developed, with an attempt to standardize the operation techniques, facilitate efficacy evaluations, minimize complications, and improve therapeutic effectiveness.
Imaging techniques
X-ray
X-ray is feasible for the peripheral lesions. X-ray guidance is simple and affordable and enables the observation of the needling pathway and the site of needle tip (whether it is at the center of the lesion). While X-ray often is less time-consuming, the localization is not sufficiently accurate and can not clearly display the vessels and organs surrounding the lesion. Currently it has gradually replaced by computed tomography (CT).
Ultrasound
Ultrasound is suitable for larger lesions closely attached to the chest wall. Ultrasound can be performed in a real-time manner and requires short operation time. However, it can not display lesion or puncture site as clearly and directly as CT and is only suitable for lesions closely attached to the chest wall.
Computed tomography (CT)
CT has a high density resolution and can provide cross-sectional view of lesions. Also, it can clearly display the relationships among heart, large vessels, and lesion, and therefore can avoid puncturing the blood vessels, lung fissure, bullae, and/or central necrotic area. It has advantages including accurate positioning, timely detection of complication, and capability for evaluating efficacy. However, it can not monitor the puncture process in a real-time manner; instead, it can not provide static cross-sectional images, and therefore repeated CT scans are required.
Other new techniques
Other new techniques including C-arm CT (17), positron emission tomography (PET), and PET-CT have also been applied.
RF electrodes
Unipolar RF electrode
It has one active electrode, along with one or more electrode pads. Other designs include multi-needle expandable type, cooled circulation type, and irrigation type.
Multi-needle expandable type
Multiple needles arranged in arrays were inserted from a trocar. A coaxial electrode, made from multiple flexible electrodes placed in 14-19 G trocar, is introduced into the tumor tissue; by using the propulsion device on the needle handle, the electrode is pushed out of the trocar to expand the arrays, thus enlarging the ablation scope. The multi-needle expandable type, with a full released diameter of 3.5 cm, can produce a 3-5 cm necrotic area and a 5-6 cm injured area.
Cooled circulation type
Using a hollow and dual-chamber design, the cooled circulation type adopts an internal cooling method, during which the cooling water is circulated to the needle tip via an electric pressure pump, so as to cool down the active electrode and prevent the drying and carbonization of tissues around the needle tip. This technique is helpful to decrease impedance and produce larger and more effective coagulative necrosis sites. The cooled circulation type can be divided into single cluster type and tri-needle cluster type, and the latter has a larger ablation area after a single operation.
Irrigation type
The tip of the irrigation type has a small hole, via which liquid (typically normal saline) can be injected into the tissue to be ablated, so as to increase the electrical conductivity and thermal conductivity of the tissue, increase the ablation area, and prevent tissue carbonization.
Bipolar RF electrodes
The bipolar RF electrodes are composed of two electrodes (including active electrode and loop electrode); alternatively, the tip of one electrode has both active electrode and loop electrode, and no loop electrode pad is required. For patients with metal implants or cardiac pacemaker, bipolar RF electrode should be applied.
All these three types can be employed in the RF ablation for lung tumors. Since patients have spontaneous breathing and large lung mobility, multi-needle expandable type is recommended to facilitate the coverage of tumors and minimize the lung injury due to the movement of RF electrode. For tumors near the heart, major vessels, trachea, and bronchus, however, single-needle types may be safer.
Indications and contradictions
Indications
Curative ablation
The curative ablation can achieve the complete necrosis of the tissues of lung tumors and may cure the condition and/or prolong the survival.
Primary lung cancer
The early-stage peripheral NSCLC (sized ≤3 cm and without lymph node metastasis or distant metastasis); patients with poor heart and lung functions; elderly patients; and/or patients refuse to receive a surgery.
Pulmonary metastases
The primary lesion has been effectively controlled; the number of unilateral lung metastases is ≤3; the number of bilateral lung metastases is ≤5; and/or maximal tumor diameter is ≤3 cm.
Palliative ablation
Palliative ablation can maximize the induction of coagulative tumor necrosis and thus achieve the purposes of lowering tumor burden and relieve symptoms.
Primary lung cancer
The tumor size is >3 cm; multiple needles, points or times of treatment can be delivered; and/or other treatments can be used in combination.
- Solitary pulmonary recurrence following a surgery for the primary lung cancer;
- Progression or relapse of lung tumor after radiochemotherapy or molecularly targeted therapy for peripheral lung carcinoma;
- Progression or relapse of lung tumor after radiochemotherapy for peripheral NSCLC;
- Peripheral lung cancer complicated with malignant pleural effusion following pleural biopsy/fixation;
- Advanced central NSCLC;
- In the event of intractable pain when ribs or thoracic vertebrae are involved, ablation of the local bone invasion can yield analgesic effects.
Pulmonary metastases
Number and size of pulmonary metastases have exceeded the limitations of curative ablation.
Contraindications
Absolute contraindications
Patients with severe bleeding tendency, platelets less than 50×109/L, and severe platelet function disorders (prothrombin time >18 s and prothrombin activity <40%). Anticoagulation and/or antiplatelet drugs should be discontinued at least 5-7 days prior to ablation.
Relative contraindications
(I) With extensive extrapulmonary metastasis and a predicted survival of <3 months; (II) with serious complications, infection, low immune function, and/or renal insufficiency; (III) with pacemaker and/or metal implants; (IV) allergic to iodine contrast medium, and therefore is unfeasible for enhanced CT scan for efficacy evaluation; and (V) with an Eastern Collaborative Oncology Group (ECOG) performance status score of >2 (Table 2).

Full table
Examinations and staging
Pre-operative examinations
Routine examinations
Routine blood, urine, and stool tests, liver/kidney function tests, coagulation function test, detection of tumor markers, blood type examination, infection screening, electrocardiogram (ECG), and lung function test are performed within 2 weeks before surgery.
Medical imaging
Chest contrast-enhanced CT, lung PET or PET-CT, abdominal ultrasound, bone scan, and skull MRI should be performed within 2-4 weeks before surgery.
Pathological examinations
Percutaneous lung puncture biopsy or bronchoscopy should be performed.
Clinical grading

Full table
Full table
Pre-operative preparation
Planning
The body position and needling pathway are determined based on the location, size, number, and shape of the tumor as well as its relationships with the heart, major vessels, trachea, and bronchus, as shown on CT or PET-CT.
Equipment and instrument
The equipment and instrument include CT machine, RF generator, RF electrode, drainage bags for thoracentesis or thoracic cavity closed drainage, ECG monitor, oxygen inhalation device, and ambulance.
Preparation of drugs
Anesthetic, analgesic, and antitussive drugs as well as drugs for hemostasis, expanding coronary artery, and lowering blood pressure should be prepared.
Patient preparation
(I) An informed consent form is signed by the patient and/or his/her family (representative); (II) the patients are fasted for food or water 4 hours before surgery; (III) skin preparation if necessary; (IV) establishment of intravenous access if necessary; (V) oral administration of an antitussive drug before surgery if necessary; and (VI) pre-operative education.
Procedures
Body position
Different body position is employed based on the tumor location. In principle, a comfortable body position with the shortest puncture distance should be used.
Disinfection and anesthesia
The skin is disinfected with povidone-iodine and ethyl alcohol and then covered with sterile towels. The puncture site was anesthetized by local infiltration of 2% lidocaine until the pleura. Conscious sedation or general anesthesia is recommended for the following conditions: (I) pediatric patients; (II) patients who can not cooperate during the procedure; (III) expected to be with a long operation time; and (IV) the tumor is close to the parietal pleura and thus may cause sharp pain. Pre-anesthesia evaluation may be based on the American Society of Anesthesiologists (ASA) grading criteria (Table 5), and only patients with a grade of ≤III can undergo RFA.

Full table
Locating and puncturing
CT is one of the most common and accurate image-guided approaches. During the procedure, the RF electrode is inserted into the target tumor via the puncture site under the guidance of CT. The target tumor should be covered during each CT scan. After the RF electrode is located at the right position, as demonstrated on the 3D CT images, ablation is performed.
Ablation
Treatment parameters are set based on the model of RF ablation system, model of RF electrode, tumor size, and relationships among tumor and its surrounding structures (The selection of lung tumor ablation parameters can be adjusted depending on the equipment manufacturer’s recommended parameters). To ensure the complete ablation of the target tumor, the range of ablation should include the target tumor and the lung tissues 0.5-1.0 cm around the tumor (i.e., the so-called “ablation area”). After the ablation, the puncture pathway should also be ablated before the withdrawal of the RF electrode to minimize tumor implantation and bleeding. Monitoring of the heart rate, blood pressure and oxygen saturation is required during the ablation process, and the patient should be closely observed for breathing, pain, cough, hemoptysis, and so on. Symptomatic treatment should be delivered when necessary.
Small tumors
Patients with ≤3 tumors sized ≤3 cm may receive a single RFA.
Middle-sized tumors
Patients with tumors sized 3-5 cm should receive single multi-focused RFA.
Large tumors
Patients with tumors sized >5 cm should receive single multi-focused RFA firstly, followed by radiotherapy or multiple multi-focused RFA.
Tumors in special sites
For lesions near the heart, major vessels, trachea, bronchus, esophagus, diaphragm, and pleural cupula, single-needle puncturing is recommended; meanwhile, the direction of the puncturing should be parallel with the major structures and maintained more than 0.5 cm.
Post-operative scan
A second CT scan should be performed immediately after the surgery to assess whether the operation is successful (whether the treatment has been completed or whether the tumor has been fully covered by the ablation). Meanwhile, any possible complication is observed.
Post-operative management
The patient is asked to take a supine position for 2-4 h, during which the vital signs are monitored. Chest X-ray or CT scan can be performed after 24-48 h to see if there are complications (such as asymptomatic pneumothorax or pleural effusion).
Complications and treatment
RF ablation is a relatively safe local treatment. Its complications are graded according to the criteria described by the International Working Group on Image-Guided Tumor Ablation under the Society of Interventional Radiology (SIR) (18) (Table 6). According to the time of occurrence, they are classified as immediate complications (≤24 h after ablation), perioperative complications (24 h to 30 d after ablation), and delayed complications (>30 d after ablation).

Full table
The complications after RF ablation for lung tumors can be categorized into puncture-related complications such as pulmonary haemorrhage, hemothorax, pneumothorax, cardiac tamponade, and air embolism and ablation-related complications such as chest pain, pleural reactions, cough, and skin burns. The case-fatality rate of RF ablation for lung tumor ranges 0-5.6% (19).
In literature with a sample size of larger than 100 cases, the case-fatality rate of RF ablation was 0-2.2%, with the incidences of severe and mild complications being 3-24.5% and 21.3-64.9%. The death causes include hemorrhage, pneumonia, worsened pulmonary fibrosis, pulmonary embolism, acute heart failure, and respiratory failure (20).
Pain
Pain is often reported using the Common Toxicity Criteria (CTC version 2.0) developed by US National Cancer Institute: 0—absent; 1—mild pain not interfering with function; 2—moderate pain interfering with function and requiring painkiller, but not interfering with activities of daily living; 3—severe pain interfering with activities of daily living and requiring painkiller; and 4—disabling pain.
Intra-operative pain
(I) Etiology: surgeries under local anesthesia are often associated with varied degrees of pain, which may be caused by the stimulation of the pleural nerve by heat conduction. In their univariate and multivariate analyses, Okuma et al. (21) found that the occurrence of pain was significantly correlated the short tumor distance from chest wall (<1 cm); (II) Treatment: if the pain is severe, complete anesthesia of the pleura is required. In some patients, the use of analgesics or even conscious sedation/anesthesia may be needed. Or, the target temperature is lowered to 70 °C before it is gradually increased several minutes later. Or, by observing the 3D CT images, the operator may find out whether the RF needle is approaching the pleura; if so, the RF needle may be rotated before ablation. Or, the RF needle may be inserted into the thoracic cavity to make the visceral pleura be apart from the parietal pleura, forming artificial pneumothorax (22,23).
Post-operative pain
The post-operative pain is usually mild (levels 1-2) and will last for several days, or 1 to 2 weeks in certain patients, which do not require special management. While moderate or above pain is rare, it can be treated by non-steroidal analgesic drugs.
Post-ablation syndrome
This may occur in approximately two thirds of patients. (I) Etiology: the severity and duration of the absorption of tumor necrosis products depend on the necrosis area and the patients’ general conditions. Post-ablation syndrome typically will not occur in patients with small lesions. The symptoms last 2-7 days in most patients, but may last 2-3 weeks in patients with larger ablated tumors; (II) Treatment: most symptoms are transient and self-limiting, and symptomatic treatment is often sufficient. In a small number of patients, non-steroidal drugs may be added. A small dose of short-term glucocorticoids would be helpful when necessary.
Pneumothorax
The incidence of pneumothorax is 5-63% (19,21). Pneumothorax may be assessed using The CTC (version 2.0): 0—no pneumothorax; 1—no intervention is needed; 2—closed thoracic drainage is needed; 3—pleural fixation or surgical treatment is needed; and 4—life-threatening.
Intra-operative pneumothorax
(I) Etiology: according to Hiraki et al. (24), the risk factors of intra-operative pneumothorax included male (with larger vital capacity), no history of breast surgery (without pleural adhesions), ablation of multiple tumors (multiple punctures), lesions in middle and lower lobes (with larger lung mobility), small and deep lesions (difficult to locate, and multiple punctures are required), large tumors (requiring multi-focused ablation, repeated punctures, cluster needle, and ablation duration >3 hours). Sano et al. (25) demonstrated that older age, use of multi-needle expandable electrode, and high-power output were significantly correlated with the development of pneumothorax; (II) Treatment: a small pneumothorax may not require management. Medium to large pneumothorax can be treated by thoracentesis or by placing the closed thoracic drainage device. It has been reported that 3.3-38.9% (mean: 11.0%) of the patients required the placement of closed thoracic drainage device (19,24) It has also been reported that pneumothorax is most frequently seen in patients with upper lobe tumors who have undergone RF ablation, which may be explained by the following reason: there is a large difference between alveolar pressure & pleural space pressure; when the patients are allowed to stand upright, a large amount of air will constantly enter the thoracic cavity; (III) Prevention: to minimize the occurrence of pneumothorax, the puncturing should be performed in a more skillful way. Rapid needling and accurate puncturing are keys to avoid multiple punctures. Meanwhile, a coaxial needle system is recommended; the normal saline or anesthetic can be injected into the pleural juncture via the injection hole, thus making the extrapulmonary tissues become thicker.
Delayed pneumothorax
The incidence of delayed pneumothorax is about 10%. Delayed pneumothorax, which is considered when it is observed 72 h after ablation, can be managed as described above.
Pleural effusion
A small amount of pleural effusion is usually observed after ablation, with an incidence rate of 1.3-60% (median: 13.4%) (19). Again, the CTC version 2.0 can be applied: 0: none; 1—asymptomatic and requires no intervention; 2—symptomatic, requiring diuretics; 3—symptomatic, requiring oxygen inhalation or therapeutic paracentesis; and 4—life-threatening (requiring tracheal intubation). (I) Etiology: the development of pleural effusion is related with pleural irritation due to high temperature during ablation. Risk factors leading to the occurrence of pleural effusions include chronic obstructive pulmonary disease, a large lesion, ablation of multiple lesions at one time, lesions near the pleura (<10 mm), and prolonged ablation (24); (II) Treatment: general observation or conservative treatment will be sufficient. In case of the presence of moderate to large pleural effusion, paracentesis/suction or closed thoracic drainage will be needed. However, the proportion of patients requiring thoracic drainage is below 10%; (III) Prevention: the ablation site should be as far as possible from the pleura.
Haemorrhage (19)
The incidence of intra-operative hemoptysis ranged 3.3-18.2% (11.1%), and the incidence of major hemoptysis is extremely low. The incidence of pulmonary hemorrhage is 0-11% (7.1%), which is inconsistent with those of hemoptysis and postoperative bloody sputum. The incidence of hemothorax is 1.9-16.7% (4.3%). (I) Etiology: no specific risk factor has been found (21). It has been found that the development of haemorrhage may be correlated with the small lesions, long puncturing pathway, co-existence of chronic obstructive pulmonary disease, and pulmonary arterial hypertension; (II) Treatment: ablation should be immediately performed once intraoperative hemoptysis occurs. Meanwhile, hemostatic agent should be intravenously infused until the hemoptysis gradually stops or decreases. Pulmonary hemorrhage can be absorbed spontaneously. Postoperative bloody sputum is often self-limiting, and can last 3-5 days. If a small amount of pleural fluid is found during the surgery, the patient should be closely observed and may receive conservative treatment. Presence of moderate to large pleural effusion suggests active bleeding, which needs to be treated by paracentesis/suction or closed thoracic drainage. Literature has demonstrated that about 10% of patients require thoracic drainage, together with the use of hemostatic drugs. In the case that conservative treatment fails, embolization or exploratory thoracotomy can be performed; (III) Prevention: since ablation itself can cause blood coagulation, the bleeding will gradually stop with the progress of ablation. As a result, the incidence of major bleeding is not high during the procedure. During the puncture, blood vessels or atelectatic lung tissue should be avoided. Blood coagulation time, platelet count, and the use of anticoagulant should be taken into consideration before the surgery.
Cough
CTC version 2.0 can be applied: 0—absent; 1—mild, relieved by non-prescription medication; 2—requiring narcotic antitussive; and 3—severe cough or coughing spasms, poorly controlled or unresponsive to treatment. (I) Etiology: severe intra-operative cough may be due to the stimulation of alveoli, bronchi, pleura after the increase of local temperature. Post-operative cough is often due to the inflammatory response caused by the local tumour tissue necrosis and the thermal injury of the surrounding lung tissue after RF ablation; (II) Treatment: cough can be alleviated immediately after oral administration of antitussive agent or injection of lidocaine via the injection hole in the RF needle. In some patients, however, the cough will not stop until the ablation is completed. Patients with post-operative cough may be appropriately administered with drugs for curing cough and reducing phlegm; (III) Prevention: oral administration of codeine 30 minutes before surgery may help to alleviate the cough response.
Pleural reaction
(I) Etiology: the ablation stimulates the vagus nerve that dominates the parietal pleura, and the vagus nerve excitement can slow down the heart rate, and even cause cardiac arrest. Also, it may because the local anesthesia is not sufficient. Some patients have limited knowledge about the disease; they may feel fearful of treatment and even in a state of high tension; (II) Treatment: when this happens, the ablation should be suspended to allow complete local anesthesia. Atropine, sedatives and other drugs should be properly applied; (III) Prevention: adequate preoperative communication may help the patients to be mentally relaxed. Or, the adjacent pleura should be completely anesthetized.
Rare complications
Other potentially fatal complications include obstinate pneumothorax due to bronchopleural fistula, air embolism, and pneumonia. Other severe complications include injury to adjacent nerves (for example, the brachial plexus, intercostal nerves, phrenic nerve, and recurrent laryngeal nerve are heat-sensitive), pericardial tamponade, tumor implantation in needling pathway, pulmonary abscess, and skin burns.
Follow-up and efficacy evaluation
Follow-up
Chest CT and detection of tumor markers should be performed again. PET-CT may be applied if feasible. It is important to evaluate whether the lesion has completely disappeared and whether there is any local progression or new lesion. PET-CT is more accurate in judging the treatment efficacy and is helpful in identifying any extra-pulmonary metastasis. Also, the quality of life, any improvement in disease condition, and survival should also be evaluated.
Changes in imaging findings in the ablation zone (26,27)
CT changes
A second agent-enhanced chest CT should be performed 4-6 weeks after the surgery, and the results shall be used as a baseline for further evaluations. Chest CT was performed every 3 months within 2 years after operation and every 6 months 2 years later.
Early phase (within 1 week)
In a successfully treated patient, the ablation zone increases, the lesion shows honeycomb-like hypodensic change, and GGO is visible around (or partially) its ring. However, GGO alone may overestimate the effectiveness of ablation. Ghost cells may be seen in this zone during the pathological examination, which may be explained by the following mechanism: the sudden coagulative necrosis of tumor and the destruction of microcirculation after heat ablation may prevent the release of intracellular lysosomal enzymes; also, the infiltration of inflammatory cells can delay the cell autolysis. Nicotinamide adenine dinucleotide diaphorase (NADH) in vivo/in vitro staining and other special staining methods shall be applied for differential diagnosis.
Intermediate phase (1 week to 3 months)
As the ablation zone increases constantly, a sharp enhanced ring may appear around the perimeter.
Late phase (after 3 months)
The ablation zone remains stable or slightly enlarges compared with baseline after 3 months. Six months later, the ablation zone remains stable or gradually shrinks, which may be shown in different morphologies (e.g., fibrosis, voids, nodules, atelectasis and disappearance), or in combination.
PET-CT changes
If condition allows, a second PET-CT may be performed 3 months after the surgery, and then every 6 months. The results can be described using standard uptake value (SUV).
Evaluation of local efficacies
Response Evaluation Criteria in Solid Tumors (RECIST) can be used for evaluating the local efficacies. The evaluation typically is performed 3 months after the RF ablation (Table 7).

Full table
Complete ablation
Complete ablation is achieved if there is any of the following findings on CT: the target tumor disappears; without cavity, solid nodules, atelectasis, or fibrosis on enhanced images. Or, PET-CT indicates that the target tumor has no radionuclide enrichment or has normal SUV value.
Incomplete ablation
Incomplete ablation is considered if any of the following occurs: incomplete cavernous formation, with some solid or liquid components remaining and contrast enhanced signs on the CT scan; partial fibrosis, with solid residues in the fibrotic lesion, which presents contrast enhanced signs on the CT scan; solid nodules with an unchanged or increased size, which presents contrast enhanced signs on the CT scan. Or, PET-CT indicates that the target tumor still has radionuclide enrichment or has abnormally high SUV value after the ablation.
Local tumor progression
Local tumor progression is considered if: CT indicates that, after the complete ablation of the target tumor, scattered, nodular, and irregular eccentric enhancement around the tumor recurs; Or, after PET-CT indicates that the target tumor has no radionuclide enrichment or has normal SUV value after the ablation, radionuclide enrichment or abnormally high SUV value recurs. A second ablation or other treatment is required in patients with local progression.
Evaluation of long-term efficacies
During the 2-5-year follow-up, Kaplan-Meier analysis or life table (survival) analysis is applied to calculate the overall survival (OS), disease-free survival (DFS), and other relevant indicators.
Postoperative comprehensive treatment
While heat sinking can protect the vessels and prevent the hemorrhage of large blood vessels, it can also contribute to the incomplete RF ablation. To address this problem, drugs may be used to reduce blood flow; also, some special strategies such as temporary occlusion of specific blood vessels, arterial embolization, and chemoembolization may be applied to reduce blood flow during the ablation (28).
The role of RF ablation combined with other treatment has become a hot topic in research on lung tumors. The combination of RF ablation with surgery (29), radiotherapy (30), chemotherapy (31), and molecularly targeted drugs (32) can increase the rate of local control and prolong the survival.
Conclusions
Recent studies have shown that, following RF ablation, early NSCLC patients who cannot tolerate surgical resection (tumor diameter ≤23 cm) have the 1-, 3- and 5-year survival rates of 90%, 70%, and 50%, respectively, and a mortality rate of less than 2%. The RF ablation has the following advantages in the treatment of lung tumors: minimal invasiveness, definite efficacy, high safety, fast recovery, relatively simple operation, and wide applicability. Such clinical evidence makes us believe that this technique will be more widely used in the multidisciplinary treatment of lung tumors in the future, and it has become a new treatment modality following surgery, radiotherapy, and chemotherapy. However, its efficacy still needs to be validated in prospective, randomized, multi-center clinical trials, and comparisons with other treatment including surgery (33,34) and stereotactic radiation therapy (35,36) will be necessary. Also, as a local minimally invasive ablation technique, RFA may be combined with other treatment (e.g., radiotherapy and systemic therapy) to improve the efficacy (The employment of chemotherapy or molecularly targeted drugs may be determined by the results of gene testing).
In summary, the effectiveness and safety of RF ablation in treating primary lung cancer have been well demonstrated in clinical trials. However, since the methodologies applied in different centers often differ, and the publication of this consensus document may facilitate the standardization of this technique during its promotion. Although the development of this consensus document was based on an extensive literature review, serious discussions, and repeated revisions, it still may have some errors and limitations. A more optimized clinical guidelines on the RF ablation that meets the real conditions of China is expected after the application of this document in clinical settings.
Acknowledgements
Authors’ contributions: BD Liu contributed this article; XY Zhi revised it. Main expert panel members: Li Xiao-Guang (Department of Radiology, Peking Union Medical College Hospital); Lu Qiang (Department of Thoracic Surgery, Tangdu Hospital, the Fourth Military Medical University); Ye Xin (Department of Oncology, Shandong Provincial Hospital, Shandong University); Li Lu (Department of Thoracic Surgery, PLA 306 Hospital); Li Hui (Department of Thoracic Surgery, Chaoyang Hospital, Capital University of Medical Sciences); Hu Bin (Department of Thoracic Surgery, Chaoyang Hospital, Capital University of Medical Sciences); Ding Jian-Yong (Department of Thoracic Surgery, Zhongshan Hospital, Fudan University); Li Chen-Rui (Department of Interventional Medicine, Cancer Institute & Hosptial, Chinese Academy of Medical Sciences); and Liu Chen (Department of Radiology, Peking University Third Hospital).
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [PubMed]
- Chen WQ, Zhang SW, Zou XN, et al. An analysis of lung cancer mortality in China, 2004 - 2005. Zhonghua Yu Fang Yi Xue Za Zhi 2010;44:378-82. [PubMed]
- She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143:1117-26. [PubMed]
- Rose SC, Dupuy DE, Gervais DA, et al. Research reporting standards for percutaneous thermal ablation of lung neoplasms. J Vasc Interv Radiol 2009;20:S474-85. [PubMed]
- Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 2000;174:57-9. [PubMed]
- Hiraki T, Gobara H, Iishi T, et al. Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg 2007;134:1306-12. [PubMed]
- Hiraki T, Gobara H, Mimura H, et al. Percutaneous radiofrequency ablation of clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2011;142:24-30. [PubMed]
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007;243:268-75. [PubMed]
- Lanuti M, Sharma A, Digumarthy SR, et al. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:160-6. [PubMed]
- Pennathur A, Abbas G, Gooding WE, et al. Image-guided radiofrequency ablation of lung neoplasm in 100 consecutive patients by a thoracic surgical service. Ann Thorac Surg 2009;88:1601-6; discussion 1607-8. [PubMed]
- Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 2008;9:621-8. [PubMed]
- Ambrogi MC, Fanucchi O, Cioni R, et al. Long-term results of radiofrequency ablation treatment of stage I non-small cell lung cancer: a prospective intention-to-treat study. J Thorac Oncol 2011;6:2044-51. [PubMed]
- Liu B, Liu L, Li Y, et al. Survival after radiofrequency ablation for 100 cases of lung neoplasms. Zhongguo Fei Ai Za Zhi 2011;14:335-9. [PubMed]
- Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol 2000;174:323-31. [PubMed]
- Beland MD, Wasser EJ, Mayo-Smith WW, et al. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology 2010;254:301-7. [PubMed]
- Lanuti M, Sharma A, Willers H, et al. Radiofrequency ablation for stage I non-small cell lung cancer: management of locoregional recurrence. Ann Thorac Surg 2012;93:921-7; discussion 927-88. [PubMed]
- Li XQ, Zhang Y, Huang DB, et al. Value of C-arm computed tomography in radiofrequency ablation of small lung lesions. Genet Mol Res 2014;13:6027-36. [PubMed]
- Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 2005;235:728-39. [PubMed]
- Zhu JC, Yan TD, Morris DL. A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol 2008;15:1765-74. [PubMed]
- Kashima M, Yamakado K, Takaki H, et al. Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center's experiences. AJR Am J Roentgenol 2011;197:W576-80. [PubMed]
- Okuma T, Matsuoka T, Yamamoto A, et al. Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Intervent Radiol 2008;31:122-30. [PubMed]
- Hiraki T, Gobara H, Shibamoto K, et al. Technique for creation of artificial pneumothorax for pain relief during radiofrequency ablation of peripheral lung tumors: report of seven cases. J Vasc Interv Radiol 2011;22:503-6. [PubMed]
- Lee EW, Suh RD, Zeidler MR, et al. Radiofrequency ablation of subpleural lung malignancy: reduced pain using an artificially created pneumothorax. Cardiovasc Intervent Radiol 2009;32:833-6. [PubMed]
- Hiraki T, Tajiri N, Mimura H, et al. Pneumothorax, pleural effusion, and chest tube placement after radiofrequency ablation of lung tumors: incidence and risk factors. Radiology 2006;241:275-83. [PubMed]
- Sano Y, Kanazawa S, Gobara H, et al. Feasibility of percutaneous radiofrequency ablation for intrathoracic malignancies: a large single-center experience. Cancer 2007;109:1397-405. [PubMed]
- Abtin FG, Eradat J, Gutierrez AJ, et al. Radiofrequency ablation of lung tumors: imaging features of the postablation zone. Radiographics 2012;32:947-69. [PubMed]
- Palussière J, Marcet B, Descat E, et al. Lung tumors treated with percutaneous radiofrequency ablation: computed tomography imaging follow-up. Cardiovasc Intervent Radiol 2011;34:989-97. [PubMed]
- Gadaleta CD, Solbiati L, Mattioli V, et al. Unresectable lung malignancy: combination therapy with segmental pulmonary arterial chemoembolization with drug-eluting microspheres and radiofrequency ablation in 17 patients. Radiology 2013;267:627-37. [PubMed]
- Shen Y, Zhong M, Jiang W, et al. Video-assisted radiofrequency ablation for pleural disseminated non-small cell lung cancer. BMC Surg 2013;13:19. [PubMed]
- Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 2006;129:738-45. [PubMed]
- Li X, Zhao M, Wang J, et al. Percutaneous CT-guided radiofrequency ablation as supplemental therapy after systemic chemotherapy for selected advanced non-small cell lung cancers. AJR Am J Roentgenol 2013;201:1362-7. [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [PubMed]
- Kim SR, Han HJ, Park SJ, et al. Comparison between surgery and radiofrequency ablation for stage I non-small cell lung cancer. Eur J Radiol 2012;81:395-9. [PubMed]
- Zemlyak A, Moore WH, Bilfinger TV. Comparison of survival after sublobar resections and ablative therapies for stage I non-small cell lung cancer. J Am Coll Surg 2010;211:68-72. [PubMed]
- Renaud S, Falcoz PE, Olland A, et al. Is radiofrequency ablation or stereotactic ablative radiotherapy the best treatment for radically treatable primary lung cancer unfit for surgery? Interact Cardiovasc Thorac Surg 2013;16:68-73. [PubMed]
- Bilal H, Mahmood S, Rajashanker B, et al. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;15:258-65. [PubMed]


