Establishment of a malignant pleural effusion mouse model with lewis lung carcinoma cell lines expressing enhanced green fluorescent protein
Abstract
Background and objective: Malignant pleural effusion (MPE) is a poor prognostic factor in patients with advanced lung cancer. The aim of this study is to establish a mouse model of MPE using Lewis lung carcinoma (LLC) cell lines expressing enhanced green fluorescent protein (EGFP).
Methods: The mouse model was created by injecting LLC-EGFP cells directly into the pleural cavity of nude mice under the guidance of stereomicroscope and then mice were sacrificed periodically. The dynamic growth and metastasis of tumor cells were screened using in vivo fluorescence imaging. The remaining mice were subjected to transverse computed tomography (CT) periodically to analyze the rate of MPE formation. The survival rate and tumor metastasis were also observed after modeling. Pleural fluid was gently aspirated using a 1 mL syringe and its volume was measured. When two or more mice bore MPE at the same time, we calculated the average volume. The correlation of MPE with the integrated optical density (IOD) were analyzed.
Results: Four days after the inoculation of LLC-EGFP cells, green fluorescence was observed by opening the chest wall. The tumor formation rate was 100%, and the IOD gradually increased after inoculation. The metastatic foci were mediastinal, contralateral pleural and pericardial. The metastasis rates were 87%, 73%, and 20%, respectively. CT imagings revealed that the rates of MPE formation on days 7, 14 and 21 were 13%, 46%, and 53%. The mean survival time of nude mice was 28.8 days. The average MPE volume increased obviously on day 10 and peaked on day 16 with a value of 0.5 mL. The MPE volume and IOD were significantly correlated (r=0.91, P<0.0001).
Conclusions: This study was the first to establish a mouse model of MPE by injecting LLC-EGFP into the pleural cavity under the guidance of a stereomicroscope. The model can enable dynamic observations of the biological behavior of tumor cells in the pleural cavity. It might be helpful for basic research on advanced lung cancer as well as anti-tumor drug development.
Key words: Malignant pleural effusion; enhanced green fluorescent protein; nude mouse model; integrated optical density
Introduction
Malignant pleural effusion (MPE) is a common cause of death in patients with advanced lung cancer and currently lacks effective clinical treatment. Therefore, it is essential to establish MPE animal models. It has been reported (1) that MPE models can be established by inoculation of tumor cells via caudal vein, intratracheally, or via pleural cavity. However, intratracheal inoculation can be associated with a high operation-related mortality rate (17%); meanwhile, the tumor cells often spread to trachea or lung but seldom reach the pleura. Therefore, the rate of MPE formation usually is low (2). When the same lung tumor cell line was injected via caudal vein or pleural cavity, the latter can result in higher rate of MPE formation and larger MPE volume, and can avoid the metastasis of tumor cells in multiple organs (3). Although it somehow differs from the spontaneous metastasis of tumor cells from a primary site to the pleural cavity, trans-pleural inoculation provides an independent mode to study a series of biological behaviors of tumor cells in pleural cavity. Green fluorescent protein (GFP) is a non-enzymatic reporter gene, after transfecting tumor cells, it can be passed on to daughter cells along with the division and growth of tumor cells and will disappear when the tumor cells die. In fact, GFP has been widely applied in transgenic research and in vivo imaging of tumors (4).
The in vivo fluorescence imaging technology, an optimal imaging of multiplexed fluorescent reporters in small animals, has provided an important tool for observing biological behaviors (including occurence, growth, metastasis, angiogenesis, and the interaction between tumor cells and host microenvironment) and evaluating the efficacy of anti-cancer drugs in a direct and objective way. However, no literature in China has reported the application of this technology in MPE models. In our current study, we tried to establish a MPE nude mouse model by trans-pleural inoculation of Lewis lung carcinoma cell lines expressing enhanced green fluorescent protein (LLC-EGFP); meanwhile, by observing the tumor growth with in vivo fluorescence imaging system. We tried to explore its biological characteristics. These fluorescent model is therefore a powerful and reliable tool with which to investigate pleural metastasis of lung cancer and pre-clinical drug development.
Materials and methods
Materials
Cell line
The LLC-EGFP cell line was purchased from American Type Culture Collection (ATCC).
Experimental animals
Totally 40 BALB/c (nu/nu) nude mice (35 males and 5 females), aged 4-6 weeks and weighing 19-27 g were used in this experiment. All the nude mice were kept and used in specific pathogen-free (SPF) barrier system [Laboratory license number: SYXK (Jiangsu) 2007-0011]. The feeding room was maintained under a 12-h light:dark cycle, at 55±10% relative humidity, and at a temperature of 22±2 ℃. These animals were fed with cobalt-60-sterilized rat/mouse diet pellets (Jiangsu Xietong Organism, China).
Equipment
The equipment used in this experiment includes: Siemens Sensation 16 Slice CT Scanner (tube voltage 120 kVp; current 93 μA), in vivo fluorescence imaging system including stereo microscope) (ZOOM645S, Yucheng Optics, China), Retiga Exi cooled digital color (Qimaging, USA), and fluorescence excitation equipment (LG-150-A, Nanjing Chaoteng Science&Technology Development Limited Corporation, China).
Methods
LLC-EGFP cell line
The cell line was cultured in RPMI-1640 complete medium containing 10% FBS in 37 ℃, 5% CO2 incubator under saturated humidity. Subculture every three days to obtain well-grown cells for experiments.
Establishment of MPE mouse models
LLC-EGFP cells were suspended in phosphate buffered saline (PBS), and the cell concentration was adjusted to 5×105/50 µL. Ketamine was used in the liquid form for intramuscular anesthesia. Mice were fixed on a plane plate in a supine position. Skin in the precordial region was disinfected. A 0.5-cm-long longitudinal excision was made 0.5 cm away from the right side of manubrium sterni, skin and subcutaneous fascia were retracted without damage of intercostal muscles. Stopped the bleeding with a cotton swab. A total of 50 µL of cell suspension was pipetted with micropipettor and injected into pleural cavity via intercostal space, during which care was taken to avoid the penetrating of blood vessels. The whole process should not be too fast. The depth of needle penetration was about 3-5 mm to avoid piercing the visceral pleura or lung. After the injection, the wound was sutured and the skin was disinfected with alcohol swab. After being awakened, the mice were placed back in their original boxes and fed with sufficient foods and water. The whole procedure was finished on aseptic benches.
Monitoring indicators
(I) The general conditions of the animals, including eating, activity, appearance, and response to external stimuli, were observed on a daily basis. (II) Observation of tumor growth: four days after inoculation, three mice were randomly killed under anesthesia every three days, with a total planned observation period of three weeks. The tumor-bearing mice were dissected and the gross findings were photoed under natural light or excitation light sources. The expression of GFP was detected using in vivo fluorescence imaging system. (III) Three days after the detection of GFP expression, all the remaining mice underwent chest CT to observe the MPE formation and calculate the rate of MPE formation at this time point; The observation was repeated on the 14th day and 21st day, and the rates of MPE formation were also calculated. A small incision was made at ear in MPE-bearing mice; the survival time was compared with these mice and those without MPE. When cachexia developed, mice were dissected to observe the tumor growth and the involvement of its neighboring organs using in vivo fluorescence imaging system. (IV) During the dissection, the abdominal cavity was exposed firstly, and the pleural fluid was aspirated (and measured) under diaphragm on both sides using a 1-mL syringe. When two or more mice bore MPE at the same time point, we calculated the average volume. Exfoliative cytology was performed for MPE smears. (V) Sections of pleural tumor tissues were stained with hematoxylin and eosin.
Chest CT
After induction of anesthesia, mice were placed in a supine position, with their heads fixed. The parameters for the scanner was as follows: collimator 64 mm × 0.6 mm, pitch 1.4, tube voltage 120 kVp, and reference tube current 93 μA. The scanned images were transmitted to a multi-function image post-processing workstation (Syngo MMWP CT workplace VA30A) in a real-time manner.
Photo-taking
The thoracic cavity was sufficiently opened. The expression of GFP was detected using in vivo fluorescence imaging system, with emission wavelength of 520 nm, excitation wavelength of 480 nm, and exposure time of 1 s.
HE staining and preparation of MPE pictures
The preparation method is based on reference 5.
Analysis of intergral optical density (IOD) using DT2000 image analysis software
The circumstance and IOD were set as the parameters. The circumstances of tumor nodules inside the thoracic cavity were measured manually. The software automatically produced the corresponding IOD values and transmitted them to an Excel table. The sum of IOD values was calculated for each mouse; three repeated measurements were performed for each mouse, and the mean values were calculated. Using the inoculation time as the x-axis and the mean MPE volume at the specific time point as the y-axis, we drew the curve of MPE formation and analyzed the correlation between the MPE volume and the IOD values in the same mouse.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 software. The correlation between the MPE volume and the IOD values in the same mouse was analyzed, and P<0.05 was considered significantly different.
Results
Observation of tumor growth using in vivo fluorescence imaging
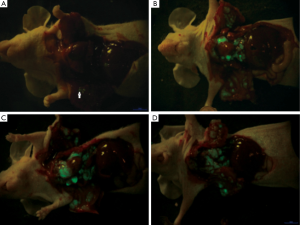
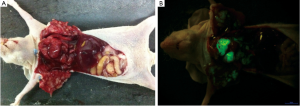
The whole surgical procedure was smooth. Only 4 mice died intra-operatively, whereas the remaining mice successfully completed the experiment. The tumor growth was observed in 21 mice. Green fluorescence was acquired in the pleural on day 4; metastasis to the contralateral pleura, hilum of lung, and mediastinal lymph nodes were observed on day 10; the size of the metastatic foci remarkably increased on day 16; and the whole chest was entirely occupied at day 25 (Figure 1).
Rate of MPE formation, survival time, and tumor metastasis
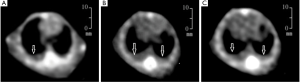
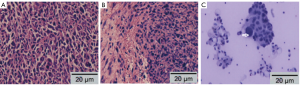
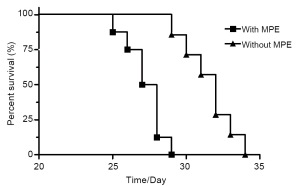
Fifteen mice were used in this section of experiment. Unilateral MPE was visible in two mice on day 7, yielding an early rate of MPE formation of 13%. The rate of MPE formation was 46% on day 14. On day 21, five mice had bilateral MPE and three had unilateral MPE on day 21, yielding a peak rate of MPE formation of 53%. Figure 2 show the CT imagines of the same mouse on days 7, 14, and 21. HE staining for sections of parietal pleural tumor tissues showed the accumulation of adenocarcinoma cells; MPE smears showed LLC cells with large nuclei and visible nucleoli (Figure 3). The mean survival time of nude mice was 28.8 days. Twenty days after implantation, mice with MPE showed remarkably decreased activities, shortness of breath, pale skin, and poor response to external stimuli; compared with those without MPE, these mice had significantly shorter survival time (Figure 4). After the tumor-bearing mice were dissected, gross observation showed that there were multiple metastatic tumor nodules on pleura or inside mediastinum, showing particle-like aggregation, grey/dark in color, and even rupture of a small number of tumors. Fluorescence microscopy provided clearer imaging of the metastatic foci, 13 mice occurred mediastinal and hilar lymph node metastasis, 11 mice occurred contralateral pleural metastasis and 3 mice developed pericardial effusion. yielding a metastasis rate of 87%, 73%, and 20%, respectively. No metastasis to liver, bone, or adrenal gland was observed (Figure 5).
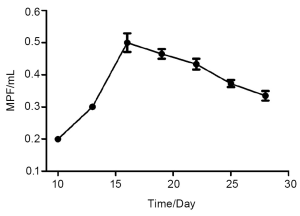
Drawing the curve of MPE formation and analyzing the correlation between the MPE volume and the IOD values
Totally 36 mice completed this experiment, and MPE was detected in 17 mice. The mean MPE volume gradually increased 10 days after inoculation and reached a peak (0.5 mL) on day 16. The volume slightly decreased later. The curve of MPE formation is basically consistent with the imaging findings. (Figure 6) Photos were taken under fluorescence tomography in vivo imaging system; the IOD values were processed using DT2000 image analysis software. Correlation analysis between the MPE volume and the IOD values showed there was a linear relationship: in fact, the the MPE volume was significantly correlated with the IOD values (r=0.91, P<0.0001) (Figure 7).

Discussion
In 1972, Stanton et al. (6) established MPE models by airway inhalation-based method; subsequently, several MPE models were developed, mainly differed in terms of the immune status of hosts, selection of tumor cells, and location/pathway of the inoculation of tumor cells. Data demonstrated that the production of MPE requires tumor cells to invade the pleura and express high levels of VEGF (3).In recent years, a prevailing way for tumor cell inoculation is to directly administered the cells via the pleural cavity. Although it somehow differs from the spontaneous metastasis of tumor cells from a primary site to the pleural cavity, it provides a useful way for research on the subsequent behaviors (including tumor angiogenesis, tumor growth, invasion, metastasis, and the formation of MPE) of tumor cells after they reach the pleural cavity. In 2005, Stathopoulos (6) developed a novel mouse model of MPE by injecting Lewis lung cancer (LLC) cells directly into the pleural space. For this model, incisions were made in skin, subcutaneous fascia, and intercostal muscles, and therefore can easily injure the intercostal artery, resulting in massive bleeding; meanwhile, because the parietal pleura is thin and fragile, penetration into the pleura can not be easily felt, and therefore it is difficult to locate the needle tip. After cell suspensions are added with indigo dye, it is not uncommon that the blue cell suspicions are not in pleural cavity; rather, they are inside mediastinum or lung. In fact, this method has poor stability, uncertain rate of tumor formation, and high animal consumption, and especially it is not feasible for beginners. Our current experiment is conducted under stereomicroscope, which clearly visualizes the beating heart, the floating lungs (due to respiration), and the intercostal artery. Thus, it provides clear direction for inserting a needle into the intercostal space. Micropipettes have certain advantages including accurate measurement and blunt tip; therefore, they are relatively easier to control the speed during the needle feeding. As a common laboratory equipment, stereo microscope is featured by low cost, simple to use, relatively low injury to the animal, which contribute to stable modeling results, good repeatability, and high rates of tumor formation and MPE formation. For the first time, we developed this mouse model of MPE under the guidance of the stereo microscope without retraction the intercostal muscle and observed, in a dynamic manner, a series of processes including the formation of tumor masses, the invasion of the surrounding tissues, and the distant metastasis. Compared with the previous MPE models, our current mode provides a chance to observe the expression of green fluorescent protein (GFP), and therefore can evaluate the growth of LLC cells inside the thoracic cavity of nude mice in a relatively objective way; thus, we can identify the micrometastases and lymph node metastasis that can not be visualized by direct observation. We believe such a method is valuable in exploring the mechanism governing the pleural metastasis of tumor cells.
It has been reported that MPE formation can be visualized under CT imaging two weeks after the inoculation of tumor cells (6,7). The volume of MPE formation and its incidence constantly change during the progression of the disease. However, no data concerning the MPE mouse model has been available. In our current study, chest CT scan was performed on day 7 after modeling and found unilateral MPE in two mice, yielding a rate of MPE formation of 13%. However, random dissection of mice on day 7 did not reveal any MPE, which can be explained by the low MPE formation rate in this early stage. Subsequently, more mice were found to be with bloody MPE, and their mean volume gradually increased as time went by and reached a peak (0.5 mL) on day 16. The highest MPE formation rate in our current study was 56%. LCC cells are a population of murine lung adenocarcinoma cells. Mice inoculated with LLC cells have a similar rate of MPE formation as patients with advanced lung adenocarcinoma. In our study, bilateral MPE formation was obtained at week-2 post-implantation, which is consistent with the previous reports. In addtion,loco-regional tumor growth and distant metastasis of these tumors occur spontaneously and rapidly throughout the pleural cavity in a manner consistent with clinical human disease.
IOD is the sum of optical densities of pixels within a user-defined area of interest. In a human hepatocellular carcinoma xenograft model in nude mice established by the Liver Cancer Institute of Fudan University, IOD showed correlation with tumor size (8). No expression of green fluorescence in the necrotic parts of a tumor, therefore, IOD expression is not only correlated with tumor size but also with its functions; in fact, IOD value is the sum of the optical densities of the functional living cells. In our experiment, detection of IOD values of the tumor nodules in thoracic cavity showed that the volume of MPE and IOD were significantly correlated. This may be explained by the mechanism of tumor angiogenesis: Most tumor cells are highly metabolically active, and they usually have higher nutritional needs to maintain their growth. Therefore, they must be able to form new blood vessels (9). However, these new blood vessels typically develop in an uncontrolled manner. Compared with the normal vessels, these new vessels have structural aberrations and fragile walls and lack smooth muscle and basement membrane. There are large gaps among endothelial cells, making the vessels more permeable. In addition, the vascular network has remarkable structural disorders, including massive blind ends, arteriovenous shunts, and local bulging, resulting in increased exudation and inter-tissue high pressure, which may also facilitate the penetration and distant metastasis of tumor cells. Furthermore, factors (e.g., VEGF) secreted by tumor cells can promote the development of tumor microvessels and increase the vascular permeability (10). The above mechanism is also one of the main causes of MPE. With the number of new vessels increases, the tumor size gradually grows, IOD rises, and the production of effusion also increases. The MPE volume and IOD increase after the inoculation; at the end-stage, the tumor tissue develops ischemia, ulceration, and necrosis, whereas the IOD and MPE volume do not further increase. Changes in IOD and MPE volume in the same mouse show certain correlation, with the new vessels as a link. Stathopoulos (6) found the correlation between the number of pleural tumor foci and the MPE volume (P<0.005). Observation of the change in IOD during the anti-angiogenic therapy in MPE subjects can indirectly reflect the change of MPE volume and thus facilitate efficacy evaluation. However, our current experiment had only limited number of nude mice; larger sample size is warranted in future studies.
Stereo microscope in our study did not find any metastasis to lung, brain, bone, and other common metastatic sites of lung cancer, which may be due to the features of fluorescent imaging. Due to the influence of viable mammalian cells and the optical absorption and scattering in tissues, current fluorescent imaging detects, at most, only 1×106 cells (11). In addition, detection for the metastasis of GFP tumor cells in a viable animal (or human) requires that the number of metastatic tumor cells is sufficiently large and the metastatic foci are located in sites that are relatively easy to detect. The metastatic foci in pleural cavity usually are small in size and often interfered by air and skin, making the fluorescence intensities outside the viable body dramatically decrease. In our pilot experiment, green fluorescence was observed in local areas via reversible skin-flaps; however, the fluorescence imaging for the distant micrometastases was unclear. The skin-flaps can expand the fluorescence route in the organ of interest. we had successfully observed the growth and metastasis of orthotopically implanted tumors in nude mice using reversible skin-flaps (12); however, for multiple micrometastases in pleural cavity, the skin-flaps could not provide sufficient fluorescence route. In addition, due to the rapid growth and metastasis of tumor in thoracic cavity, frequent opening of skin flaps can cause severe damage to the nude mice. Some nude mice died before the distant metastasis could be observed; under such situation, the mice was killed and their pleural cavity were opened for direct observation. According to literature, a new protein called Katushka has been isolated. It is the brightest known protein with emission at wavelengths longer than 620 nm. By making whole-body imaging more sensitive owing to reduced absorption by tissues and less scatter, rapid maturation, high pH stability, and high optical stability (13). this new protein can be used for non-invasive dynamic imaging of numerous cellular processes that occur even in deep tissues (pleural cavity) in animals.
In summary, we successfully established a MPE nude mouse model by the trans-pleural inoculation of LLC-EGFP. Also, by observing GFP expression and MPE formation, we investigated the characteristics of the pleural invasion and metastasis of lung cancer cells and explored the correlation of MPE with IOD. By mimicking a series of biological behaviors of lung cancer that invades pleura at its late stages in a real-time manner, we tried to provide a scientific and feasible platform for studies on the medical treatment of lung cancer accompanied with MPE.
Acknowledgements
This Chinese edition of this article was published in Chinese Journal of Lung Cancer. Translated with permission from the Chinese Journal of Lung Cancer.
Disclosure: The authors declare no conflict of interest.
References
- Stathopoulos GT, Kalomenidis I. Animal models of malignant pleural effusion. Curr Opin Pulm Med 2009;15:343-52.
- Mase K, Iijima T, Nakamura N, et al. Intrabronchial orthotopic propagation of human lung adenocarcinoma--characterizations of tumorigenicity, invasion and metastasis. Lung Cancer 2002;36:271-6.
- Yano S, Shinohara H, Herbst RS, et al. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol 2000;157:1893-903.
- Chaudhuri TR, Mountz JM, Rogers BE, et al. Light-based imaging of green fluorescent protein-positive ovarian cancer xenografts during therapy. Gynecol Oncol 2001;82:581-9.
- Stathopoulos GT, Zhu Z, Everhart MB, et al. Nuclear factor-kappaB affects tumor progression in a mouse model of malignant pleural effusion. Am J Respir Cell Mol Biol 2006;34:142-50.
- Stanton MF, Wrench C. Mechanisms of mesothelioma induction with asbestos and fibrous glass. J Natl Cancer Inst 1972;48:797-821.
- Stathopoulos GT, Kollintza A, Moschos C, et al. Tumor necrosis factor-alpha promotes malignant pleural effusion. Cancer Res 2007;67:9825-34.
- Yang BW, Liang Y, Xia JL, et al. Biological characteristics of fluorescent protein-expressing human hepatocellular carcinoma xenograft model in nude mice. Eur J Gastroenterol Hepatol 2008;20:1077-84.
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182-6.
- Hoffman RM. Imaging tumor angiogenesis with fluorescent proteins. APMIS 2004;112:441-9.
- Troy T, Jekic-McMullen D, Sambucetti L, et al. Quantitative comparison of the sensitivity of detection of fluorescent and bioluminescent reporters in animal models. Mol Imaging 2004;3:9-23.
- Wei S, Sun Y, Yang Z, et al. Establishment of orthotopic lung cancer model expressing enhanced green fluorescent protein. Zhongguo Fei Ai Za Zhi 2010;13:670-5.
- Hoffman RM. A better fluorescent protein for whole-body imaging. Trends Biotechnol 2008;26:1-4.


