
Toxicity after volumetric modulated arc therapy for lung cancer: a monocentric retrospective study
Introduction
With 228,150 new cases expected for 2019, lung cancer is the leading cancer killer in the United States (1). Concomitant chemoradiotherapy (cChRT) is the standard of care for locally-advanced lung cancer (2), but the addition of chemotherapy (ChT) to radiotherapy (RT) is known to increase acute toxicity. Indeed, in the study of Parashar and al, the incidence of grade 2 or higher radiation pneumonitis was significantly associated with the addition of ChT (62.7% versus 15.8%, P<0.001) in patients treated with three-dimensional conformal (3D)-RT (3). While metastasis-free survival and overall survival (OS) were significantly improved with the addition of durvalumab (4,5), acute and late toxicities remain one of the main concerns in patients treated with RT for localized lung cancers.
Intensity-modulated RT (IMRT) is now widely implemented and has replaced classical RT (3D-RT) in many tumor sites, as it allows a better target dose conformity and a better sparing of organs a risk (OAR) without compromising tumor control (6-8). This higher conformation using IMRT is possible at the expense of a volume increase in adjacent organs receiving doses in the lowest range. This “low-dose bath” may theoretically increase toxicity in the adjacent healthy tissues, especially in the lungs, despite being not really clinically reported (9-11). In a retrospective cohort of 73 patients treated with hypofractionated IMRT (2.2–2.75 Gy/fraction), severe pneumonitis and esophagitis (grade ≥3) occurred in only 7% and 1% of the population, respectively (12). For volumetric modulated arc therapy (VMAT), this low dose bath, reflected by the V5% to the lungs, has raised even more concerns. In addition to the higher theoretical risk of developing secondary malignancy (13,14), the rate of radiological pneumonitis may be higher in patients treated with VMAT compared with conformal 3D-RT (15,16). However, reported results are contradictory in terms of occurrence of lung complications following IMRT or VMAT (17,18).
Published clinical data on outcome and toxicity using VMAT in lung cancer outside clinical trial are still scarce, especially in patients receiving concomitant ChT. We aimed to report acute and late pulmonary and oesophageal toxicities in a cohort of patients with lung cancer and treated with VMAT with or without ChT at our institution.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-406).
Methods
Patients and treatment characteristics
All consecutive patients treated with (chemo-)RT using VMAT delivered with curative intent for lung cancer between November 2015 and January 2018 at the University Hospital of Brest were included. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the CHRU Brest Hospital (IRB 29BRC20.0154). All patients consented for the use of their clinical data for scientific purpose.
Prior to treatment initiation, patients underwent a total body 18F-fluorodeoxyglucose positron emission tomography combined with low dose computed tomography (18FDG PET-CT) and magnetic resonance imaging (MRI) or CT-scan of the brain for tumour staging purposes. All patients had pulmonary function tests before RT initiation. Patients in good clinical condition (performance status of 0 to 1) received cChRT. Patients with impaired performance status (i.e., ≥2) underwent RT alone. When bulky masses were present and considered as non-eligible to upfront irradiation, induction ChT was the treatment of choice, followed by cChRT. In case of persistent toxicity after induction ChT, RT alone was administered. RT was delivered using VMAT on a Truebeam Novalis STX (RapidArc©, Varian, United States) using two arcs of 6 MegaVolt.
Treatment planning
All patients had a PET/CT for the RT planning. Planning CT consisted of an intravenous contrast-enhanced CT scan in treatment position acquired from the third cervical vertebral to the upper abdomen (including a slow CT-scan of the primary tumour) which was then transferred to the Pinnacle planning system (Version 9.10; Philips Radiation Oncology Systems, Fitchburg, WI).
The gross tumour volume (GTV) consisted of the primary tumour and metastatic lymph nodes (confirmed by histopathological examination and/or FDG-PET positive) outlined on the planning CT after registration with the diagnostic FDG-PET. The clinical target volume (CTV) enclosed the GTV of the primary tumour (19) and positive lymph nodes (20) with margins defined by the Radiation Therapy Oncology Group (RTOG) recommendations depending on the histology (20-22). Planning target volumes (PTVs) were created by an isotropic 5 mm expansion of the CTVs. To generate dose-volume histogram (DVH) data, the lungs and the oesophagus (from the lower border of the cricoid cartilage to the gastro-esophageal junction) were manually delineated. The spinal cord was considered to be at the inner margin of the entire bony thoracic spinal canal.
The prescribed dose to the PTV was 66 Gy in 33 daily fractions, 60 Gy in 30 fractions in case of small cell lung cancer, or less depending on the treatment planning and dose to organs at risk. Usual dose constraints were considered: V30Gy <20%, V20Gy <30%, V13Gy <40%, V10Gy <45%, and V5Gy <65% for the lungs (23), V40Gy to the heart <30% (24) and V60Gy to the esophagus <33% (25), VxGy being the percentage of the organ receiving x Gy.
Assessment of toxicity and therapeutic outcome
During the course of radiation delivery, acute esophageal toxicity (AET) and pulmonary toxicity (APT) were assessed weekly by the treating radiation oncologist using the Common Terminology Criteria for Adverse Events (CTCAE) v.4. Follow-up visits were planned at one month and every three months thereafter for the first two years. Thoraco-abdominal-pelvic CT-scan was performed at one month following RT completion and every three months for two years thereafter. Late pulmonary and esophageal toxicities (LPT and LET, respectively) were also scored using the CTCAE v.4 from the fourth months following RT completion and every 3 months.
To distinguish ChT and RT toxicities, toxicities specifically due to ChT were also collected during and after the radiation delivery. Here, we only report toxicities due to RT. Indeed, the ChT toxicity profile is significantly different with, mainly and depending on the ChT regimen, systemic toxicities such as gastro-intestinal (nausea, …), haematological (neutropenia, anemia, …), renal and neurologic (neuropathy). In the cChRT setting, ChT is used as a radio-sensibilization agent. Therefore, toxicities are often due to the RT but increased by ChT.
Data collection and statistical analysis
All medical records were retrospectively reviewed. The following parameters were extracted from the treatment planning system: PTV volume; for the homolateral and contralateral lung mean lung dose (DMean), maximum dose (DMax), V5Gy, V10Gy, V13Gy, V20Gy, V30Gy; for both lungs: V13Gy, V20Gy, V30Gy, DMax and DMean; for the esophagus: V5Gy, V10Gy, V20Gy, V30Gy, V40Gy, V50Gy, V60Gy and maximum esophagus dose; for the heart: mean dose, V40Gy, V30Gy.
Association of clinical characteristics and dosimetric parameters with the occurrence of grade (G) ≥2 toxicity (AET, LET, APT and LPT) was evaluated with univariate analyses using the Receiver Operative Characteristics approach (area under the curve: AUC, sensitivity: Se, specificity: Sp), calculated with Medcalc 14.8.1. Multivariate analysis (MVA) using logistic regression was performed on pre-selected significant clinical and dosimetric features. A p-value below 0.05 indicated statistical significance.
All CT-scan during the follow-up were reviewed and the aspect compatible with radiation induced injuries were collected at 1, 3, 6, 9, and 12 months after RT, and described as “alveolar opacities”, “ground glass” and/or “fibrosis”. Then these radiation induced lung diseases (RILD) were noted according to their locations: around the tumour, in the same lobe as the tumour, in the same lung, in the contralateral lung or in both lungs. The impact of dosimetric and clinical parameters on the occurrence of RILD was also studied.
OS, progression-free-survival (PFS) and local control (LC) were also reported using the Kaplan Meier method.
Results
Population
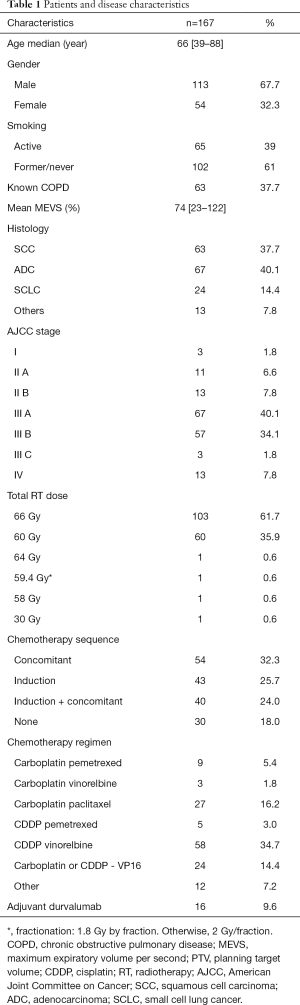
One hundred and sixty-seven patients were included. Patients’ characteristics are listed in Table 1. Median age was 66 years (range, 39–88 years). According to the American Joint Committee on Cancer (AJCC) 2017 classification 1.8% (n=3), 6.6% (n=11), 7.8% (n=13), 40.1% (n=67), 34.1% (n=57), 1.8% (n=3) and 7.8% (n=13) patients presented with a staged I, IIA, IIB, IIIA, IIIB, IIIC, and IV disease, respectively. Among them, 37.7% (n=63) had a squamous cell carcinoma, 40.1% (n=67) an adenocarcinoma, 14.4% (n=24) a small cell lung carcinoma and 7.8% (n=13) had other histologies.
Full table
Treatments characteristics
Median PTV volume was 270 cc (14.2–1,408 cc). Median radiation dose was 66 Gy (range, 30–66 Gy). The dose was reduced to 60 Gy in 60 patients either because of a small cell histology (n=24) or due to violation of normal tissue constraints (n=36). Three patients received less than 60 Gy due to major disease progression or death. Most patients (82%) received ChT. Many different regimens were used, depending on histology, patient’s performance status and past history and comorbidities. Overall, 54 patients (32.3%) patients received cCRT, 43 (25.7%) had sequential RT, 40 (24.0%) were treated with induction ChT followed by cChRT and 30 (18.0%) had exclusive RT (Table 1).
Dosimetric parameters are summarised in Table S1. Median V20Gy and V30Gy to both lungs were 23.0% (18.3–28.0%) and 14.4% (9.7–18.3%), respectively. Median V60Gy to the oesophagus was 8.7% (5.4–11.3%) and median dose to the heart 8.5 Gy (0.11–30.5 Gy).
Acute and late pulmonary and oesophageal toxicities
The G ≥2 APT, AET, LPT, and LET toxicity rates for the entire cohort were respectively 22.2%, 30.0%, 16.8% and 5.4%. Grade 3 and above APT, AET and LPT remained relatively rare with respective rates of 3%, 6.6% and 3%. No grade ≥3 LET occurred.
Esophageal toxicity
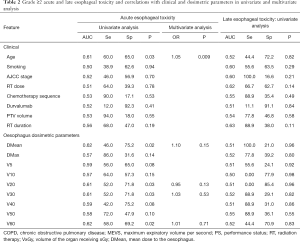
AET G ≥2 was observed in 5 (25%), 18 (33.3%), 16 (31.4%) and 11 (32.4%) patients after exclusive RT, cChRT, sequential RT and induction ChT followed by cChRT, respectively. On univariate analysis, only age, DMean, V30 and V60 to the esophagus with respective threshold of 27.5 Gy, 43% and 12.4% were significantly associated with a risk of AET >G2 (Table 2). On MVA, only age remained significant (P=0.03). Patients older than 67 years were twice more likely to present a grade ≥2 AET than younger patients, with respective rates of 42.3% and 20.9%.
Full table
Overall, occurrence of severe LET remained low: only 9 patients (5.4%) developed G ≥2 toxicity (respectively, 1, 5 and 3 the cCRT, sequential, and induction + cChRT groups). On univariate analysis, no clinical or dosimetric features achieved a significant correlation (Table 2).
Pulmonary toxicity
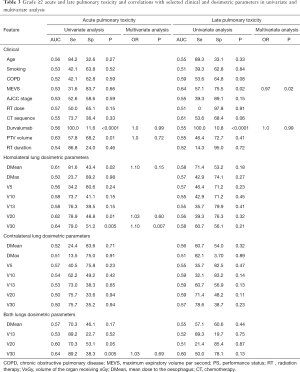
The rates of APT did not significantly vary according to the modality of ChT administration. On univariate analysis (Table 3), PTV volume and V30 (with a cut off of 22.2 Gy) to the homolateral lung were significantly associated with increased G ≥2 APT. Only the V30 to the homolateral lung remained statistically correlated with G ≥2 APT on MVA (P=0.007). Patients with a V30 to the homolateral lung >22.2 Gy were 3 times more likely to present a grade ≥2 APT than patients with V30 below 22.2 Gy, with respective rates of 33.3% vs. 11%.
Full table
The rate of severe LPT was relatively low: 13.2% patients developed a grade 2 LPT, 2.4% a grade 3 and only 0.6% developed a grade 4 LPT. To be noted, one patient died following treatment related pulmonary toxicity (grade 5 LPT) in the Induction-cChRT group. All > grade 2 LPT occurred in a ChT setting (1 G3 in the cChRT group, 2 G3 and 1 G4 in the sequential group and 2 G3 in the Induction-cChRT group).
On univariate analysis, only the pre-radiotherapy MEVS was associated with the occurrence of G ≥2 LPT. No association with ChT regimen nor dosimetric features was statistically relevant (Table 3).
RILD
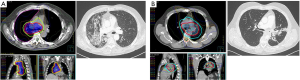
Considering all types of RILD, the rates of contralateral radiological injuries observed on CT-scan were 5.4% at one month, 5.4% at 3 months and 1.0% at 1 year, while the rate of bilateral RILD were 9.0% and 3.2%, respectively. The RILD during the follow-up at 3 and 12 months are reported in Table S2. Figure 1 illustrates examples of RILD that occurred following VMAT and the corresponding dosimetry. Given the low number of events at 9 and 12 months, association of clinical and dosimetric factors with the occurrence of RILD was only tested at 3 and 6 months, and no correlation was found.
Treatment outcomes
Median follow-up was 14.0 months (range, 0.4–47.8). The 1-year LC was 83.3% for the entire cohort, with respective values of 91.2%, 85.2%, 76.1%, 71.8% in the cChRT, sequential, induction + cChRT and RT only groups. No statistically significant differences in LC between the groups were observed (Figure S1). Among the 167 patients, the 1-year PFS was 49.2% (Figure S2). At last follow-up, 60 patients had died, all due to disease. The 1-year OS was 70.7% (Figure S3). As expected, patients with a small cell histology had poorer PFS and OS outcomes.
Discussion
VMAT has the advantage of delivering the dose to the tumour in a 360 degrees rotation in less than two minutes. But, the increase in normal tissue volume receiving low dose radiation has raised some concern, supported by conflicting clinical results. While Mc Grath and colleagues showed that VMAT was better than 3D-RT at sparing lung (V20Gy, V12.5Gy, V10Gy, V5Gy) compared with 3D-RT (26), opposite results have been published by Ong et al., who found higher lung dosimetric parameters (V20Gy and V5Gy) with VMAT compared with 3D-RT (27). Toxicity outcomes following VMAT-based RT for lung cancer lack, thus our work.
With 22.8%, APT, 30.0% AET, 16.8% LPT and 5.4% LET, the grade ≥2 toxicity rates observed in our cohort are in line with previous published reports focusing on IMRT (9,18,28). Data from the RTOG 0617 study that compared the use of 3D-RT to IMRT in 482 patients showed indeed that IMRT was associated with fewer G3 pneumonitis compared to the 3D-RT technique (7.9% vs. 3.5%, P=0.039) and with a reduced risk of radiation pneumonitis in adjusted analyses (OR, 0.41; 95% CI, 0.17–0.99; P=0.046). Doses to the heart, especially the V40Gy was also lower using IMRT (P<0.05). On the contrary, the lung V5Gy was not correlated with any G3 toxicity (29). On the contrary, Ling et al. did not show any difference in terms of acute toxicity between IMRT and 3D-RT in a retrospective series of 145 patients, but there was a trend toward lower rates of G ≥2 pneumonitis among IMRT patients compared to 3D-RT patients (5.4% vs. 23.0%, P=0.065) (28).
Very few data on VMAT-related toxicity in patients treated for lung cancer are however currently available in the literature (Table S3). Based on a 77-patient cohort, Wu reported low grade ≥2 toxicities with respective rates of G ≥2 APT and AET of 28.6% and 18.2%. No data on late toxicities were however reported (30) and association between acute toxicities and clinical or dosimetric features was not tested.
The largest VMAT cohort (278 patients), focusing on lung toxicity only, accounted for a ≥ G2 radiation pneumonitis rate of 7.6%. Unfortunately, the overall population’s characteristics (PTV volume, AJCC stage, …) being unavailable, comparison with our cohort is not possible (31).
Acute esophageal toxicity rates of IMRT in the literature seem to be higher than the ones we observed here with VMAT, with rates ranging between 45% and 72% (32-35). In a study comparing toxicity and outcome between IMRT and VMAT in 188 patients treated for advanced stage NSCLC, APT and severe late toxicities were however similar to ours: the rate of G2 APT (23.9%) in patients treated with IMRT was not significantly different from the rate reported in patients treated using VMAT (18.8%) (18). Compared to IMRT, the risk of developing a G ≥2 AET after VMAT was also higher in this study, but the authors attributed it to the higher percentage of patients receiving cChRT in the VMAT group.
Concomitant ChT was not associated with a higher risk of toxicity in our study. cCRT is the standard of care in patients with NSCLC (33), but ChT is known to have a radiosensitizing effect resulting in enhanced mucosal toxicity when combined with RT (3,34,35). In a metanalysis, Palma et al. found that age (>65) and administration of concomitant carboplatin/paclitaxel ChT were predictive factors for radiation induced lung toxicities (15). Similarly, in a cohort of lung cancer patients predominantly treated with concurrent carboplatin/paclitaxel, both cChRT use and age were associated with a large increase in acute lung toxicities risk (63% versus 16%), with a trend toward increased risk in patients receiving carboplatin/paclitaxel specifically. Pneumonitis occurred in 77% of patients aged 61–70, with lower rates in other patients (3). In our cohort, toxicity rates were significantly lower than the ones reported in patients treated with 3D-CRT, possibly thanks to the higher tumour-conformation and thus lower oesophagus and lungs dose profiles, erasing concomitant ChT as a potential toxicity factor. In total, one can think VMAT could enhance cChRT tolerance without compromising tumour control (6-8).
On MVA, adjuvant durvalumab was not correlated with any form of acute or late toxicities in our study. Adjuvant durvalumab has recently been shown to increase PFS and OS (4,5), but only a small subgroup of patients received durvalumab in our cohort (9.6%). The rate of grade ≥2 AET in this sub-population (43.8%) was higher than in the rest of the population (28.5%), despite not reaching statistical significance (P=0.32). Anti-PD-L1 related pneumonitis is now well described and occurs in around 5% of patients with anti-PDL1, as observed in the Keynote 024 (36). But, no data on AET are available in patients treated with durvalumab specifically.
As we experienced, differentiating expected RILD from recurrence, infection and others lung diseases is difficult (37). Radiation induced inflammatory events that occurred after radiation therapy are likely to result in scannographic modifications (38) but data are missing in the literature regarding the radiological semiology in this context. To our knowledge, no study has established a correlation between dosimetric parameters (especially low doses such as V5Gy, V10Gy, and V13Gy) and the radiological appearance of the lung parenchyma on CT-scan after radiation. But, the contralateral and bilateral injuries we observed here may be the direct reflection of this modern irradiation technique.
Besides the drawbacks of retrospective studies, some other limitations of our work should be noted. Firstly, no control group treated with 3D-RT or IMRT is available. Secondly, the present cohort is heterogeneous in terms of histology, RT dose, and ChT modality. Thirdly, the limited follow-up makes the interpretation of late toxicity rates difficult although we could argue that the aggressiveness and poor PFS of the disease makes long-term complications difficult to assess and that the majority of events usually occur during the first year after treatment (39).
Conclusions
The low rates of pulmonary and esophageal toxicity observed in our cohort of patients treated with arc therapy for lung cancer show that the use of arc therapy appears to be a safe irradiation technique. Larger prospective studies are needed, ideally with respiratory function tests during the follow-up, to analyse the clinical consequence of the VMAT technique on respiratory parameters. Such a study is currently ongoing at our institution (NCT03931356). Moreover, the radiological semiology of pneumonitis induced by immunotherapy and/or induced by VMAT also needs to be further studied.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-406
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-406
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-406
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-406). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the Ethics Committee of the CHRU Brest Hospital (IRB 29BRC20.0154). All patients consented for the use of their clinical data for scientific purpose.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Parashar B, Edwards A, Mehta R, et al. Chemotherapy significantly increases the risk of radiation pneumonitis in radiation therapy of advanced lung cancer. Am J Clin Oncol 2011;34:160-4. [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Grills IS, Yan D, Martinez AA, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 2003;57:875-90. [Crossref] [PubMed]
- Christian JA, Bedford JL, Webb S, et al. Comparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007;67:735-41. [Crossref] [PubMed]
- Bezjak A, Rumble RB, Rodrigues G, et al. Intensity-modulated radiotherapy in the treatment of lung cancer. Clin Oncol (R Coll Radiol) 2012;24:508-20. [Crossref] [PubMed]
- Khalil AA, Hoffmann L, Moeller DS, et al. New dose constraint reduces radiation-induced fatal pneumonitis in locally advanced non-small cell lung cancer patients treated with intensity-modulated radiotherapy. Acta Oncol 2015;54:1343-9. [Crossref] [PubMed]
- Shi A, Zhu G, Wu H, et al. Analysis of clinical and dosimetric factors associated with severe acute radiation pneumonitis in patients with locally advanced non-small cell lung cancer treated with concurrent chemotherapy and intensity-modulated radiotherapy. Radiat Oncol 2010;5:35. [Crossref] [PubMed]
- Zhang J, Yu XL, Zheng GF, et al. Intensity-modulated radiotherapy and volumetric-modulated arc therapy have distinct clinical advantages in non-small cell lung cancer treatment. Med Oncol 2015;32:94. [Crossref] [PubMed]
- Jaksic N, Chajon E, Bellec J, et al. Optimized radiotherapy to improve clinical outcomes for locally advanced lung cancer. Radiat Oncol 2018;13:147. [Crossref] [PubMed]
- Chao PJ, Lee HF, Lan JH, et al. Propensity-score-matched evaluation of the incidence of radiation pneumonitis and secondary cancer risk for breast cancer patients treated with IMRT/VMAT. Sci Rep 2017;7:13771. [Crossref] [PubMed]
- Abo-Madyan Y, Aziz MH, Aly MM, et al. Second cancer risk after 3D-CRT, IMRT and VMAT for breast cancer. Radiother Oncol 2014;110:471-6. [Crossref] [PubMed]
- Palma DA, Senan S, Haasbeek CJ, et al. Radiological and clinical pneumonitis after stereotactic lung radiotherapy: a matched analysis of three-dimensional conformal and volumetric-modulated arc therapy techniques. Int J Radiat Oncol Biol Phys 2011;80:506-13. [Crossref] [PubMed]
- Teoh M, Clark CH, Wood K, et al. Volumetric modulated arc therapy: a review of current literature and clinical use in practice. Br J Radiol 2011;84:967-96. [Crossref] [PubMed]
- Jiang X, Li T, Liu Y, et al. Planning analysis for locally advanced lung cancer: dosimetric and efficiency comparisons between intensity-modulated radiotherapy (IMRT), single-arc/partial-arc volumetric modulated arc therapy (SA/PA-VMAT). Radiat Oncol 2011;6:140. [Crossref] [PubMed]
- Wijsman R, Dankers F, Troost EGC, et al. Comparison of toxicity and outcome in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapy using IMRT or VMAT. Radiother Oncol 2017;122:295-9. [Crossref] [PubMed]
- Giraud P, Antoine M, Larrouy A, et al. Evaluation of microscopic tumor extension in non-small-cell lung cancer for three-dimensional conformal radiotherapy planning. Int J Radiat Oncol Biol Phys 2000;48:1015-24. [Crossref] [PubMed]
- Grills IS, Fitch DL, Goldstein NS, et al. Clinicopathologic analysis of microscopic extension in lung adenocarcinoma: defining clinical target volume for radiotherapy. Int J Radiat Oncol Biol Phys 2007;69:334-41. [Crossref] [PubMed]
- Chapet O, Kong FM, Quint LE, et al. CT-based definition of thoracic lymph node stations: an atlas from the University of Michigan. Int J Radiat Oncol Biol Phys 2005;63:170-8. [Crossref] [PubMed]
- Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest 1997;111:1718-23. [Crossref] [PubMed]
- Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. [Crossref] [PubMed]
- Rudra S, Al-Hallaq HA, Feng C, et al. Effect of RTOG breast/chest wall guidelines on dose-volume histogram parameters. J Appl Clin Med Phys 2014;15:4547. [Crossref] [PubMed]
- Kim TH, Cho KH, Pyo HR, et al. Dose-volumetric parameters of acute esophageal toxicity in patients with lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2005;62:995-1002. [Crossref] [PubMed]
- McGrath SD, Matuszak MM, Yan D, et al. Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: A dosimetric and treatment efficiency analysis. Radiother Oncol 2010;95:153-7. [Crossref] [PubMed]
- Ong CL, Verbakel WF, Cuijpers JP, et al. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010;97:437-42. [Crossref] [PubMed]
- Ling DC, Hess CB, Chen AM, et al. Comparison of Toxicity Between Intensity-Modulated Radiotherapy and 3-Dimensional Conformal Radiotherapy for Locally Advanced Non-small-cell Lung Cancer. Clin Lung Cancer 2016;17:18-23. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Wu K, Xu X, Li X, et al. Radiation pneumonitis in lung cancer treated with volumetric modulated arc therapy. J Thorac Dis 2018;10:6531-9. [Crossref] [PubMed]
- Rades D, Glatzel E, Werner EM, et al. Prevalence and Characteristics of Symptomatic Pneumonitis After Radiotherapy of Patients With Locally Advanced Lung Cancer. Anticancer Res 2019;39:6909-13. [Crossref] [PubMed]
- Wang J, Zhou Z, Liang J, et al. Intensity-Modulated Radiation Therapy May Improve Local-Regional Tumor Control for Locally Advanced Non-Small Cell Lung Cancer Compared With Three-Dimensional Conformal Radiation Therapy. Oncologist 2016;21:1530-7. [Crossref] [PubMed]
- Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010;28:2181-90. [Crossref] [PubMed]
- Werner-Wasik M, Yorke E, Deasy J, et al. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys 2010;76:S86-93. [Crossref] [PubMed]
- Wijsman R, Dankers F, Troost EG, et al. Multivariable normal-tissue complication modeling of acute esophageal toxicity in advanced stage non-small cell lung cancer patients treated with intensity-modulated (chemo-)radiotherapy. Radiother Oncol 2015;117:49-54. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Benveniste MF, Welsh J, Godoy MC, et al. New era of radiotherapy: an update in radiation-induced lung disease. Clin Radiol 2013;68:e275-90. [Crossref] [PubMed]
- Graves PR, Siddiqui F, Anscher MS, et al. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol 2010;20:201-7. [Crossref] [PubMed]
- Chen C, Uyterlinde W, Sonke JJ, et al. Severe late esophagus toxicity in NSCLC patients treated with IMRT and concurrent chemotherapy. Radiother Oncol 2013;108:337-41. [Crossref] [PubMed]


