
Participation in lung cancer screening
Following publication of the Dutch-Belgian NELSON trial, which confirmed the findings of the US National Lung Screening Trial (NLST) published a decade earlier, that low radiation dose CT (LDCT) screening reduces lung cancer mortality, many countries are planning to introduce screening programmes for lung cancer targeted to people at high risk of the disease. The success of such programmes depends on a number of factors that have been extensively researched and several resultant position statements are available to guide those implementing programmes (1-3). However, some issues remain a pressing concern because there is no clear consensus on how they should be addressed. Informed uptake, participation, and adherence (to successive screening rounds) are related issues that impact on the equity and cost-effectiveness of a programme as well as the overall impact on lung cancer mortality. It is also important to recognise that choosing not to participate in lung cancer screening is a legitimate choice, although evidence suggests that a decision not to participate is often uninformed (4). In this article we discuss the participation rates observed in some clinical trials and in pilot programmes and review the factors that may influence uptake, drawing on evidence from other screening programmes and interventions in similar target populations.
Participation rates and inequalities in lung cancer screening
Participation in screening programmes for cervical, breast and colorectal cancer is around 75%, 70% and 60% respectively in the UK (5-9). This is the rate of uptake of the offer and attendance at the screening test (for breast and cervical cancer) or return of the test (colorectal cancer). In lung cancer screening, it is the proportion of people that attend the assessment appointment. The proportion who actually undergo the LDCT is often also quoted. The rationale for this is that there is a further check on eligibility in the screening assessment process which is not a measure of participation. Uptake is lower in socioeconomically deprived groups (10) and among current smokers (11), where the highest rates of lung cancer are seen. This is important because currently, LDCT is only offered to those at high risk of lung cancer for whom the risk-benefit ratio is favourable. The evidence from clinical trials in lung cancer screening is limited because only one has used a true population approach in recruitment, the United Kingdom Lung Screen pilot trial (UKLS) (12), and offering screening as part of a clinical trial may underestimate the participation rates of a real-world service (13). In the UKLS trial, 31% of eligible people responded to an initial questionnaire but only 11.5% of participants were at high enough risk for trial entry and 47% of these gave their consent. In the NELSON trial a population approach was initially used for adult males only. Thirty-two percent of those eligible responded to a questionnaire on general health, lifestyle, and smoking history (which did not mention the NELSON trial) (14). Nineteen percent of the respondents met the eligibility criteria for the trial and received an invitation for participation in the trial, an information leaflet, and an informed consent form combined with a short questionnaire (14). Of these individuals, 51% gave informed consent and were recruited. The lower participation rate in the UKLS may in part be explained by the eligibility criteria that required a higher risk of lung cancer than in NELSON.
However, in both well-conducted trials, current smokers at higher risk were less likely to participate than former smokers (15-17). A more detailed analysis showed that female sex, older age, and more deprived socioeconomic background were independent determinants of reduced participation in UKLS (18). Similarly in NELSON, which recruited mostly men (84%), participants were younger and had a higher level of education than eligible non-responders, and also reported better health and increased physical activity (19). Indeed, these same demographic and smoking-related inequalities in participation have been observed internationally across several other lung screening trials, including the NLST (20) and the Danish Lung Cancer Screening Trial (21).
The participation rate of people at higher risk was low enough to be of concern for the successful implementation of lung cancer screening programmes. Where a more conventional approach to recruitment has been employed, participation rates have so far been low. In the US, where LDCT screening has been funded since 2015 (22), participation rates were 3.3% of the eligible population in 2015 and more recently estimated to be 14% in 2018 although only 4% in the uninsured (23,24). That this effect was observed among socioeconomically deprived groups (25) is not unexpected; echoing previous work in smoking cessation (26,27) as well as a pervasive social gradient in uptake of established population screening programmes for other cancer types (10). Research into high-risk groups’ attitudes to lung cancer screening has identified potential psychological barriers to taking part; most frequently reported by individuals of a socioeconomically deprived background. These included a nihilistic view of lung cancer which was perceived as an uncontrollable disease with attitudes of fatalism, self-blame, low perceived treatment efficacy and stigma (28,29).
However, it is critical that future programmes engage high-risk groups equitably, to ensure this population benefits and to avoid exacerbating existing inequalities in lung cancer mortality. The health gains from screening the more deprived group are not well-defined; in both NLST and NELSON, participants were better educated, younger, and less likely to be current smokers compared to background population smoking rates (30) or non-responders (17) respectively. Whilst individuals from socioeconomically deprived backgrounds may have higher frequencies of competing causes of death, they are also more likely to be current smokers, so the potential health gain from LDCT screening and smoking cessation may be greater. Participation rates amongst women are lower than men in most trials and pilots (12,30), yet the reduction in mortality is greater in women (31,32). Further work may be needed that focuses on factors which improve uptake in women.
In an effort to enhance informed participation in lung cancer screening, there has been a recent focus on pilot programmes targeted within socioeconomically deprived areas of the UK. Pilot screening programmes in Liverpool, Manchester, Leeds, Nottingham, and London have concentrated recruitment efforts in deprived areas (32-36). Only the London-based Lung Screen Uptake Trial (LSUT) has reliably measured participation rate amongst people identified through primary care records and used a randomised controlled real world demonstration design to ascertain uptake in the service context (36). This demonstration pilot showed a participation rate of 53%, of which 91% of those eligible chose to be screened, comparing favourably with uptake of bowel cancer screening in similar populations (37). In the other pilots participation rate was difficult to measure, but whether fixed site (Liverpool) or mobile (Manchester and Nottingham) scanners were used, 35–53% of eligible attendees subsequently participated in LDCT screening across all pilots. All of these pilots identified ever smokers potentially at risk of lung cancer and the first contact was from the primary care doctor.
Improving participation in cancer screening
While evidence for strategies that improve participation in lung cancer screening is relatively limited, useful insight may be drawn from previous research in other screening programmes or other healthcare interventions in similar populations. Interventions that are consistently associated with improved participation include the use of pre-invitation letters, scheduled appointments, and reminder letters (38-40). In particular, the use of social media messaging and text reminders in prompting cancer screening engagement remains an area of active research.
Primary care endorsement has been shown to have a modest effect on uptake of colorectal screening of 6–7% (41,42), although this did not appear to change the socioeconomic gradient. In colorectal screening, uptake in the Scottish and Australian programmes was increased by 24 and 23% respectively through the use of pre-notification letters (38,43). In the Scottish programme, this was true for all socioeconomic groups. Provision of enhanced procedural information prior to colorectal cancer screening was associated with improved uptake rates of between 0 and 6% (41,42). Reminder letters were found to increase colorectal screening uptake by 7% overall and by 11% in deprived groups, indicating some impact on the socioeconomic gradient (42). Breast cancer screening uptake was increased by 19% by reminder letters sent a few days prior to appointment for a mammogram (44). Indeed, Duffy and colleagues’ rapid review of interventions designed to improve cancer screening participation found reminders (postal, text and telephone) were consistently associated with higher uptake; with letters achieving the largest increase (relative to telephone) and personalised letters for non-participants being particularly effective (39). Pre-scheduled appointments have been shown to increase rates of attendance 3-fold in breast cancer screening (45) and timed second appointments for non-attenders resulted in 20% attending for screening, contributing a 6% overall increase in screening uptake (46). In LSUT, a reminder letter providing a second pre-scheduled appointment increased uptake of lung cancer screening among non-responders by 24% (36). In addition, recorded delivery letters and incentives, including financial, are associated with improved participation (47).
Understanding and improving participation in lung cancer screening
Optimising informed participation rates in lung cancer screening remains a key priority for successfully implementing effective and equitable programmes. According to the ‘COM-B (Capability, Opportunity, Motivation – Behaviour) system’ framework, which is the central component of Michie and colleagues’ ‘Behaviour Change Wheel’ (Figure 1) (48), there are three necessary conditions for any given behaviour: capability (the individual’s psychological and physical capacity to engage in a behaviour), opportunity (the physical and social resources needed to enact a behaviour) and motivation (the individual’s cognitive and affective processes that direct behaviour). These conditions are proposed to interact to determine whether a behaviour is enacted. For example, if opportunity were high and motivation were low, an individual may still engage in a behaviour because it was made easy to do so. On the other hand, if opportunity were low and motivation were high, an individual may persist with a difficult behaviour because they felt strongly motivated. In both scenarios, these individuals’ behaviour may be thwarted if their capability (actual or perceived) were low.
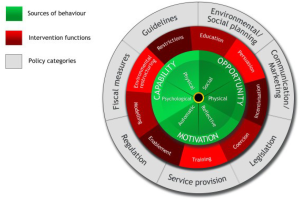
With regards to opportunity and capability, almost half of non-participants surveyed (n=748) in UKLS identified practical barriers relating to travel and comorbidities (18). Comorbidities are relatively more common among the screening-eligible population compared with the general population due to the adverse health effects of long-term smoking and social deprivation being associated with increased rates of disability. Qualitative research in the US with those opting out of lung cancer screening also reported practical concerns; including the inconvenience of the screening location and the time taken to travel and have the scan alongside full-time employment (49). In another US study, the potential cost of screening was a further practical concern, particularly among participants of an ethnic minority background (50).
Research into high-risk groups’ attitudes to lung cancer screening has also identified potential psychological factors that may undermine an individual’s motivation to take part. These included a nihilistic view of lung cancer which was perceived as an uncontrollable disease with attitudes of fatalism, self-blame, low perceived treatment efficacy, stigma, and in the US, distrust of the healthcare system (28,29,51). A fifth of those declining participation in UKLS reported emotional barriers reflecting concern about their risk of lung cancer (affective risk perception), and fear and avoidance of lung cancer information; most frequently among current smokers (18). Population-based surveys within the UK have shown avoidant beliefs about lung cancer screening and an intention not to be screened were associated with fatalism, low perceived treatment efficacy and survival benefit, and not being willing to have surgery for a screen-detected early stage lung cancer (52,53).
Ways in which to further increase participation, using these insights as well as evidence-based strategies from screening programmes for other cancer types, were tested in the LSUT. The intervention invitation materials were designed to be ‘targeted, stepped and low burden’; with targeted content aiming to minimise fear, fatalism and stigma and to provide a low burden level of information prior to the appointment (54). There was no overall difference in participation rate between the intervention materials and control invitations. However, uptake was improved among those with the highest levels of deprivation which importantly provides evidence for a reduced social gradient, and participation rates in both arms of the trial were significantly higher than has previously been observed (36). This may reflect the application of the measures supported by existing evidence in both arms of the trial, designed to improve opportunity and capability to reduce non-intentional barriers to participation across both arms. These included primary care invitations, pre-notification letters, scheduled appointments, and reminders (with a second scheduled appointment). Participant invitations also framed the screening offer within a broader ’Lung Health Check’ including other lung tests (e.g., spirometry) rather than framing the offer solely as a cancer screening appointment, which aimed to minimise fear. There was also no upfront mention of smoking cessation which may disengage an important minority of current smokers (55).
Much work has been done on improving engagement with people living in socioeconomically deprived communities, where a combination of practical measures that improve opportunity and tailored engagement also seem to be effective. These involve the invitation and reminder process as discussed above but also more practical issues such as the siting of the CT scanner and travel. The successful pilots in Manchester and Liverpool ensured easy access to scanners.
Future strategies to increase engagement in lung cancer screening may operate at multiple levels, ranging from lung screening awareness messages delivered at population level using targeted mass media and social media campaigns, to community health educators facilitating uptake in local target populations, to paper-based or digital tailored invitation materials designed to encourage engagement at an individual level. Such interventions must, however, be carefully developed and evaluated with principles of co-production in mind. Where population data are limited, true for most countries without an established national primary care database, there may be difficulties identifying people who are at high risk and therefore potentially eligible for screening. Here, a method of identifying geographical regions with a higher incidence of lung cancer may allow effective targeting of engagement strategies. If this is combined with invitation methods that are effective in the social sector, participation rates may be improved. There are a number of sociodemographic “segmentations” that may allow this and this approach has been used to identify and target health interventions in COPD and congestive heart failure (56).
The majority of benefit in cancer screening programmes is derived from continued involvement in the programme. This is because most cancers are found during the incidence screening rounds. It is therefore essential that participation in subsequent screening rounds in the programme is maximised. Similar approaches to those used in initial engagement therefore need to be employed to support continued participation and further research is needed to establish the key factors that improve adherence.
Conclusions
There is much work to do to ensure that LDCT screening programmes deliver their full potential to improve lung cancer outcomes. Improving informed participation is one of the most important elements in realising this potential. With the COVID-19 pandemic likely to exacerbate inequalities in lung cancer outcomes (57), it is imperative to commence planning for strategies to mitigate impact on lung cancer early diagnosis.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the guest editor (Witold Rzyman) for the series “Implementation of CT-based screening of lung cancer” published in Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-917
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-917). The series “Implementation of CT-based screening of lung cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. DRB reports personal fees from Astra Zeneca, personal fees from Roche, personal fees from MSD, personal fees from BMS, from Johnson and Johnson, outside the submitted work. Dr. KB reports personal fees from Astra Zeneca, outside the submitted work. Dr. SQ reports grants from Cancer Research UK, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oudkerk M, Devaraj A, Vliegenthart R, et al. European position statement on lung cancer screening. Lancet Oncol 2017;18:e754-66. [Crossref] [PubMed]
- Kauczor HU, Baird AM, Blum TG, et al. ESR/ERS statement paper on lung cancer screening. Eur Respir J 2020;55:3277-94. [Crossref] [PubMed]
- Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest 2015;147:295-303. [Crossref] [PubMed]
- van den Bergh KA, Essink-Bot ML, van Klaveren RJ, et al. Informed participation in a randomised controlled trial of computed tomography screening for lung cancer. Eur Respir J 2009;34:711-20. [Crossref] [PubMed]
- Logan RF, Patnick J, Nickerson C, et al. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439-46. [Crossref] [PubMed]
- Programmes NCS. NHS Breast Screening Programme. Annual Report 2011. In: NHS, editor. 2011. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/breast-screening-programme/breast-screening-programme-england-2011-12
- Altobelli E, Lattanzi A, Paduano R, et al. Colorectal cancer prevention in Europe: burden of disease and status of screening programs. Prev Med 2014;62:132-41. [Crossref] [PubMed]
- Altobelli E, Lattanzi A. Breast cancer in European Union: an update of screening programmes as of March 2014 Int J Oncol 2014;45:1785-92. (review). [Crossref] [PubMed]
- Cervical screening standards data report 1 April 2018 to 31 March 2019. Public Health England Publications. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/856927/Cervical_screening_standards_data_report_2018_to_2019.pdf
- Hirst Y, Stoffel S, Baio G, et al. Uptake of the English Bowel (Colorectal) Cancer Screening Programme: an update 5 years after the full roll-out. Eur J Cancer 2018;103:267-73. [Crossref] [PubMed]
- Bryan L, Westmaas L, Alcaraz K, et al. Cigarette smoking and cancer screening underutilization by state: BRFSS 2010. Nicotine Tob Res 2014;16:1183-9. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. UK Lung Cancer RCT Pilot Screening Trial: baseline findings from the screening arm provide evidence for the potential implementation of lung cancer screening. Thorax 2016;71:161-70. [Crossref] [PubMed]
- Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer 2008;112:228-42. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- McRonald FE, Yadegarfar G, Baldwin DR, et al. The UK Lung Screen (UKLS): demographic profile of first 88,897 approaches provides recommendations for population screening. Cancer Prev Res (Phila) 2014;7:362-71. [Crossref] [PubMed]
- Field JK, Duffy SW, Baldwin DR, et al. The UK Lung Cancer Screening Trial: a pilot randomised controlled trial of low-dose computed tomography screening for the early detection of lung cancer. Health Technol Assess 2016;20:1-146. [Crossref] [PubMed]
- Yousaf-Khan U, Horeweg N, van der Aalst C, et al. Baseline Characteristics and Mortality Outcomes of Control Group Participants and Eligible Non-Responders in the NELSON Lung Cancer Screening Study. J Thorac Oncol 2015;10:747-53. [Crossref] [PubMed]
- Ali N, Lifford KJ, Carter B, et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: a mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015;5:e008254. [Crossref] [PubMed]
- Hestbech MS, Siersma V, Dirksen A, et al. Participation bias in a randomised trial of screening for lung cancer. Lung Cancer 2011;73:325-31. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010;102:1771-9. [Crossref] [PubMed]
- Malmqvist J, Siersma V, Thorsen H, et al. Did psychosocial status, sociodemographics and smoking status affect non-attendance in control participants in the Danish Lung Cancer Screening Trial? A nested observational study. BMJ Open 2020;10:e030871. [Crossref] [PubMed]
- Verma V, Beriwal S. Medicare Approves Coverage for Lung Cancer Screening: The Case for Symptomatic Screening. JAMA Oncol 2015;1:1027-8. [Crossref] [PubMed]
- Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol 2017;3:1278-81. [Crossref] [PubMed]
- Zahnd WE, Eberth JM. Lung Cancer Screening Utilization: A Behavioral Risk Factor Surveillance System Analysis. Am J Prev Med 2019;57:250-5. [Crossref] [PubMed]
- Schutte S, Dietrich D, Montet X, et al. Participation in lung cancer screening programs: are there gender and social differences? A systematic review. Public Health Rev 2018;39:23. [Crossref] [PubMed]
- Murray RL, Bauld L, Hackshaw LE, et al. Improving access to smoking cessation services for disadvantaged groups: a systematic review. J Public Health (Oxf) 2009;31:258-77. [Crossref] [PubMed]
- Bauld L, Judge K, Platt S. Assessing the impact of smoking cessation services on reducing health inequalities in England: observational study. Tob Control 2007;16:400-4. [Crossref] [PubMed]
- Quaife SL, Marlow LAV, McEwen A, et al. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect 2017;20:563-73. [Crossref] [PubMed]
- Quaife SL, Winstanley K, Robb KA, et al. Socioeconomic inequalities in attitudes towards cancer: an international cancer benchmarking partnership study. Eur J Cancer Prev 2015;24:253-60. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Pinsky PF, Church TR, Izmirlian G, et al. The National Lung Screening Trial: results stratified by demographics, smoking history, and lung cancer histology. Cancer 2013;119:3976-83. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Crosbie PA, Balata H, Evison M, et al. Implementing lung cancer screening: baseline results from a community-based 'Lung Health Check' pilot in deprived areas of Manchester. Thorax 2019;74:405-9. [Crossref] [PubMed]
- Liverpool Healthy Lung Programme – Second year Evaluation Report. 2018. Available online: https://www.liverpoolccg.nhs.uk/media/3245/final-lhlp-2nd-year-report-10-july-2018-with-logos.pdf
- . . Available online: http://www.cancerresearchuk.org/health-professional/early-diagnosis-activities/ace-programmeAccelerate Coordinate Evaluate (ACE) Programme. 2017.
- Quaife SL, Ruparel M, Dickson JL, et al. Lung Screen Uptake Trial (LSUT): Randomized Controlled Clinical Trial Testing Targeted Invitation Materials. Am J Respir Crit Care Med 2020;201:965-75. [Crossref] [PubMed]
- von Wagner C, Baio G, Raine R, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol 2011;40:712-8. [Crossref] [PubMed]
- Libby G, Bray J, Champion J, et al. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen 2011;18:24-9. [Crossref] [PubMed]
- Duffy SW, Myles JP, Maroni R, et al. Rapid review of evaluation of interventions to improve participation in cancer screening services. J Med Screen 2017;24:127-45. [Crossref] [PubMed]
- Ghimire B, Maroni R, Vulkan D, et al. Evaluation of a health service adopting proactive approach to reduce high risk of lung cancer: The Liverpool Healthy Lung Programme. Lung Cancer 2019;134:66-71. [Crossref] [PubMed]
- Hewitson P, Ward AM, Heneghan C, et al. Primary care endorsement letter and a patient leaflet to improve participation in colorectal cancer screening: results of a factorial randomised trial. Br J Cancer 2011;105:475-80. [Crossref] [PubMed]
- Wardle J, von Wagner C, Kralj-Hans I, et al. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. Lancet 2016;387:751-9. [Crossref] [PubMed]
- Cole SR, Smith A, Wilson C, et al. An advance notification letter increases participation in colorectal cancer screening. J Med Screen 2007;14:73-5. [Crossref] [PubMed]
- Allgood PC, Maxwell AJ, Hudson S, et al. A randomised trial of the effect of postal reminders on attendance for breast screening. Br J Cancer 2016;114:171-6. [Crossref] [PubMed]
- Ore L, Hagoel L, Shifroni G, et al. Compliance with mammography screening in Israeli women: the impact of a pre-scheduled appointment and of the letter-style. Isr J Med Sci 1997;33:103-11. [PubMed]
- Hudson S, Brazil D, Teh W, et al. Effectiveness of timed and non-timed second appointments in improving uptake in breast cancer screening. J Med Screen 2016;23:160-3. [Crossref] [PubMed]
- Edwards P, Roberts I, Clarke M, et al. Methods to increase response rates to postal questionnaires. Cochrane Database Syst Rev 2007.MR000008. [Crossref] [PubMed]
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6:42. [Crossref] [PubMed]
- Carter-Harris L, Brandzel S, Wernli KJ, et al. A qualitative study exploring why individuals opt out of lung cancer screening. Fam Pract 2017;34:239-44. [Crossref] [PubMed]
- Jonnalagadda S, Bergamo C, Lin JJ, et al. Beliefs and attitudes about lung cancer screening among smokers. Lung Cancer 2012;77:526-31. [Crossref] [PubMed]
- Carter-Harris L, Ceppa DP, Hanna N, et al. Lung cancer screening: what do long-term smokers know and believe? Health Expect 2017;20:59-68. [Crossref] [PubMed]
- Quaife SL, Vrinten C, Ruparel M, et al. Smokers' interest in a lung cancer screening programme: a national survey in England. BMC Cancer 2018;18:497. [Crossref] [PubMed]
- Smits SE, McCutchan GM, Hanson JA, et al. Attitudes towards lung cancer screening in a population sample. Health Expect 2018;21:1150-8. [Crossref] [PubMed]
- Quaife SL, Ruparel M, Beeken RJ, et al. The Lung Screen Uptake Trial (LSUT): protocol for a randomised controlled demonstration lung cancer screening pilot testing a targeted invitation strategy for high risk and 'hard-to-reach' patients. BMC Cancer 2016;16:281. [Crossref] [PubMed]
- Stevens C, Smith SG, Quaife SL, et al. Interest in lifestyle advice at lung cancer screening: Determinants and preferences. Lung Cancer 2019;128:1-5. [Crossref] [PubMed]
- Doos L, Uttley J, Onyia I, et al. Mosaic segmentation, COPD and CHF multimorbidity and hospital admission costs: a clinical linkage study. J Public Health (Oxf) 2014;36:317-24. [Crossref] [PubMed]
- Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet 2020;395:1243-4. [Crossref] [PubMed]


