Novel therapies in small cell lung cancer
Introduction
Small cell lung cancer (SCLC) is an aggressive cancer of neuroendocrine origin, accounting for approximately 15% of all lung cancer cases. At the time of diagnosis, approximately 30% of the patients have limited stage disease (LS) with tumor confined to one hemithorax. The majority of the patients have extensive stage disease (ES) with metastasis beyond one hemithorax at the time of diagnosis. The incidence of SCLC in US has steadily declined over past 30 years presumably because of decrease in the percentage of smokers and change to low-tar filter cigarettes (1). Current standard of care for LS-SCLC is concurrent chemoradiation with cisplatin and etoposide for four cycles with thoracic radiation administered preferably early during the first two cycles of chemotherapy. The role for surgery is limited to a very small percentage of patients with very small peripheral primary tumors. Prophylactic cranial irradiation (PCI) should be considered in patients with LS disease and good response to chemoradiation. Management of ES-SCLC includes chemotherapy with a platinum agent (cisplatin or carboplatin) with etoposide for four cycles in the US while irinotecan is often combined with a platinum agent in Japan. Topotecan is the only FDA approved second line therapy in ES disease. Despite improvements in the imaging modalities, techniques and delivery of thoracic radiation, as well as supportive care, prognosis of SCLC remains poor, with 5-year overall survival (OS) rate of 5-10% necessitating exploration of novel therapies (2).
Genetic complexity and basis of targeted therapy in SCLC
SCLC is a heterogeneous disease with a complex genomic landscape likely resulting from chronic tobacco exposure. Genomic sequencing analyses of SCLC cell lines have revealed thousands of mutations, some of which can be therapeutically targeted. In 2010, a SCLC cell line, NCI-H209 was sequenced revealing 22,910 somatic mutations including 134 in coding exons (3). In addition, this analysis showed defects in the DNA repair pathways including transcription coupled repair and expression-linked repair. Several other studies have also identified high prevalence of inactivating mutations in TP53 (75-90%) (4), RB1 (60-90%) (5,6), and PTEN (2-4%) (7), while activating mutations have been identified in PIK3CA, EGFR and KRAS (8-10). In addition, amplification of MYC family members, EGFR and BCL2, as well as loss of RASSF1A, PTEN and FHIT (6,11) have also been described. In another report by Peifer et al., sequencing of 29 SCLC exomes, 2 genomes, and 15 transcriptomes, found an extremely high mutation rate of 7.4±1 protein-changing mutations per million base pairs. In addition to inactivation of TP53 and RB1, recurrent mutations in CREBBP, EP300, MLL, PTEN, SLIT2, and EPHA7 as well as amplifications of FGFR1 tyrosine kinase gene were also identified (12). Although many of these genetic alterations can be viewed as potential therapeutic targets in SCLC, a distinction remains to be made between the driver mutations and passenger mutations in order to determine which targets will yield a meaningful therapeutic benefit.
Since p53 inactivation is found in more than 50% of the human cancers including SCLC, several attempts have been made to restore the tumor suppressor function of p53. These include gene therapy using viruses to deliver p53 to cancer cells, synthetic peptides that stabilize and upregulate wild type p53, as well as small molecules to target key signaling interactions involving mutant p53 (13). Several of these agents have proven to have antitumor effects in pre-clinical studies and are in early clinical trials.
The complexity of genetic alterations along with the heterogeneity of SCLC phenotypes with the presence of both neuroendocrine and epithelial characteristics possibly explains the prevalence of more than one clone in any given tumor and the high rate of relapse after initial response to chemotherapy (14). Intuitively, the genetic alterations that confer resistance to conventional therapy can also serve as potential therapeutic targets.
Unsuccessful attempts at targeted therapies and anti-angiogenic agents in SCLC
Multiple studies over past 2 decades have evaluated a plethora of targeted agents alone and in combination with conventional chemotherapy in the treatment of SCLC. These agents include various tyrosine kinase inhibitors (TKIs) such as EGFR TKIs, BCR-ABL TKIs, as well as mTOR inhibitors, all of which have failed to demonstrate a survival benefit in SCLC.
SCLC cells exhibit increased levels of vascular endothelial growth factor (VEGF), which likely enables their invasive, and angiogenic potential, however, the results of clinical trials evaluating antiangiogenic agents such as bevacizumab, thalidomide, and sorafenib have been disappointing with no improvement in OS (Table 1).
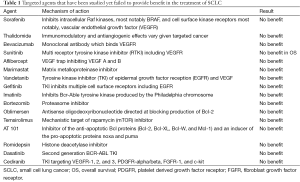
Full table
Bevacizumab is the most studied of the VEGF inhibitors. Two phase 2 trials evaluated its role in the maintenance setting in LS-SCLC. In the initial trial, response rates and progression free survival (PFS) favored bevacizumab, but subsequent trials showed increased risk of development of tracheoesophageal fistula with bevacizumab in this setting (15,16). Studies evaluating bevacizumab in combination with platinum based chemotherapy in ES-SCLC showed improvement in PFS in a very select patient population, but no improvement in median OS was demonstrated (17). Another study evaluating bevacizumab in combination with paclitaxel in chemosensitive ES-SCLC failed to show any improvement in clinical outcomes (18).
Recently published data of cancer and leukemia group B (CALGB) 30504, a phase II placebo controlled trial, exploring the role of maintenance sunitinib in ES-SCLC, showed that maintenance sunitinib after chemotherapy with platinum agent and etoposide improved median PFS by 1.6 months, but no improvement in OS was seen (19).
Aflibercept is an angiogenesis inhibitor with a unique mechanism of action. It binds to VEGFA and B thereby preventing their binding to the cell receptors. Topotecan with or without Aflibercept in platinum-treated ES-SCLC was investigated in a phase II trial which showed improvement in 3-month PFS in platinum refractory disease but not in platinum sensitive disease. However, no improvement in OS was noted in either group (20).
The failure of anti-angiogenic agents in improving OS in SCLC further reinforces the fundamental difference in the biology of SCLC from NSCLC, where bevacizumab has been shown to improve survival and is FDA approved in the first line setting in combination with carboplatin and paclitaxel (21). SCLC has therefore been the graveyard of drug development and represents an extremely challenging malignancy to treat. The large number of somatic mutations in combination with the heterogeneity of SCLC are likely major contributing factors to the failure of targeted therapies.
Targeted therapies under study in SCLC
Our knowledge of the biology of SCLC has significantly grown in recent years but this understanding is yet to be translated to successful novel therapies that can be used in clinical practice. We will summarize a number of targets in this paper. These targeted agents have been studied employing various strategies including in the maintenance or relapsed setting.
Targeting DNA repair pathways
PARP inhibition
In 2012, Byers et al. performed proteomic analysis of 34 SCLC and 74 NSCLC cell lines using reverse-phase protein arrays (RPPA) to identify differences in key oncogenic proteins and pathways in SCLC and NSCLC. Several different protein targets and downstream pathways were analyzed (22).
Consistent with prior studies, this study found higher expression of c-Kit, Bcl-2, and stathmin in SCLC. Similarly, total and phospho-Rb levels were relatively low and E2F1 expression was relatively high in SCLCs, as compared with NSCLC lines. In addition, it was also found that a few not previously described targets were also overexpressed in SCLC. These included thymidylate synthase which might explain the lack of activity of pemetrexed in SCLC. Several DNA repair and apoptosis proteins were also found to be overexpressed. Notably, mean levels of total PARP1 (a DNA repair protein and E2F1 co-activator) were 2.06-fold higher in SCLC cell lines than in NSCLC cell lines. Since PARP1 was expressed at the highest relative levels among the DNA repair proteins, this was further investigated as a potential therapeutic target in vitro. PARP inhibitors AZD2281 and AG014699 led to growth inhibition of SCLC cell lines. In addition, higher PARP1 levels correlated with significantly greater sensitivity to PARP inhibitors.
Similarly, Cardnell et al. demonstrated in vitro inhibition of SCLC proliferation by PARP inhibitor BMN 673 on SCLC cell lines and xenografts. Sensitivity to BMN 673 was associated with elevated baseline expression levels of several DNA repair proteins, whereas interestingly, greater drug resistance was observed in SCLC models with baseline activation of the PI3K/mTOR pathway (23).
These results are encouraging and indicative of potential use of PARP inhibitors in treatment of SCLC, however, whether this in vitro benefit is of clinical significance remains to be proven with clinical trials. In a phase I study of BMN 673 that included 23 SCLC patients the majority of whom had been previously treated with platinum, 2 patients had partial response, while 3 had prolonged stable disease lasting ≥24 weeks suggesting single agent activity in some patients with SCLC (24).
RAD51 inhibition
MP470 (amuvatinib) is an oral multi-kinase inhibitor that suppresses RAD51, inhibits mutant c-KIT, and PDGFR alpha. In preclinical studies, it was found to have synergistic activity with DNA damaging agents such as topoisomerase inhibitors. It was recently studied in a phase IB trial in combination with conventional chemotherapy in a variety of solid tumors (25). About 12% partial responses were seen, with majority being in neuroendocrine tumors, SCLC, and NSCLC. However, a recent phase 2 study of amuvatinib in combination with platinum-etoposide in SCLC did not show clinically meaningful improvement in PFS or OS (26). Nevertheless, it is unclear if RAD51 inhibition noted with amuvatinib is an off-target effect of this agent and it is possible that RAD51 remains an important target in SCLC but more specific inhibitors are needed to determine that.
Targeting cell cycle regulation and RNA transcription
Cyclin dependent kinase (CDK) inhibition
Roniciclib (BAY 1000394) is an oral pan-CDK inhibitor which has been shown to have antitumor activity and strong synergism with chemotherapy (cisplatin/etoposide) in SCLC models (27). A phase I trial (NCT01188252) showed that roniciclib is well tolerated with most common side effects being GI toxicity, dyspnea and thrombosis. There were no partial or complete responses per RECIST criteria, but 29% disease control rate was observed in SCLC patients. This has led to an ongoing phase II trial of roniciclib in combination with cisplatin or carboplatin with etoposide (NCT02161419).
Targeting developmental pathways
Achaete-scute family BHLH transcription factor 1 (ASCL1)
ASCL1 is a lineage oncogene providing therapeutic target for high grade neuroendocrine lung cancers. Preclinical studies have shown that ASCL1 is highly overexpressed in neuroendocrine lung cancers but is not amplified or mutated. ASCL1 knockdown mice failed to develop SCLC suggesting that it is a master regulator in SCLC development. ASCL1 is necessary for survival of high grade NE tumors which makes it a possible therapeutic target. By combining whole-genome microarray expression analysis performed on lung cancer cell lines with ChIP-Seq data designed to identify conserved transcriptional targets of ASCL1, several downstream genes were identified which can be used as potential therapeutic targets, with BCL2 being one of them. In addition, drugs that provide upstream down regulation of ASCL1 (MAPK activators) can induce apoptosis of the cell lines and can also be exploited as potential therapies (28).
Hedgehog (Hh) pathway
Hh is a critical developmental pathway during embryonic development. It is relatively quiescent in adult cells except for tissue maintenance and repair. Preclinical studies have shown upregulation of Hh pathway in many cancer cell lines including SCLC and cancer stem cells from many tumor types have been found to be sensitive to Hh pathway inhibitors (29). In addition, it has been found in preclinical studies that activation of Hh pathway in SCLC cells is independent of lung microenvironment. It has been shown that constitutive activation of Hh signaling molecule smoothened (Smo) in Rb1 and Trp53 mutant mice promoted the initiation and progression of SCLC. Conversely, deletion of Smo in these mice led to strong suppression of the development of SCLC. Furthermore, therapy with Hh pathway inhibitor after conventional chemotherapy was found to be effective in preventing SCLC recurrence in mice models (30). A recent phase I trial of Hh pathway inhibitor—sonidegib (LDE225) as a single agent in advanced solid tumors—demonstrated acceptable safety profile. Most of the responders had basal cell carcinoma and medulloblastoma (31). A three arm phase II study (ECOG 1508) evaluating the role of Hh inhibitor vismodegib in previously untreated ES-SCLC randomly assigned patients to receive either cisplatin plus etoposide (CE) or CE with either vismodegib or cixutumumab (insulin like growth factor-1 receptor inhibitor). There was no significant improvement in response rate, PFS or OS with addition of either agent to CE (32). Hh inhibitors are still in quite early phase trials for SCLC but these initial results are disappointing.
Notch pathway
The Notch pathway serves as an essential mediator in stem cell self-renewal, proliferation and differentiation (33). SCLC cell lines have shown high Notch pathway activation (34). Tarextumab (TRXT, OMP59R5) is a fully human IgG2 antibody that targets Notch2/Notch3 and blocks ligand dependent signaling. In patient-derived SCLC xenografts, TRXT in combination with chemotherapy significantly delayed tumor recurrence compared with chemotherapy combined with placebo. A phase I/II study of tarextumab in combination with six cycles of cisplatin and etoposide in ES-SCLC followed by tarextumab maintenance (PINNACLE, NCT01859741) is currently underway. Phase I results show that TRXT is generally well tolerated, and most common side effects are diarrhea, fatigue, and nausea. Several biomarkers including NOTCH receptors expression, gene signatures, and expression of target genes such as Hes1 will also be evaluated in this study (35).
Targeting cell proliferation pathways
SOX-2
In 2012, Rudin et al. analyzed 80 human SCLCs using next generation sequencing and found 26,406 somatic mutations including 7,154 missense, 536 nonsense, 12 stop loss, 243 essential splice site, 32 protein-altering insertion and/or deletion, 2,674 synonymous, 11,460 intronic and 4,295 other types (36). In addition, several members of the SOX family of genes were mutated in SCLC. SOX2 amplification was found in ~27% of the samples. Suppression of SOX2 using shRNAs blocked proliferation of SOX2-amplified SCLC lines implying that SOX2 inhibition can be used as a potential therapeutic target for SCLC. Thus far, there have been no published data on development or use of SOX2 inhibitors in SCLC.
Cell signaling pathways
c-MET receptor tyrosine kinase
c-MET and its ligand hepatocyte growth factor (HGF) regulate multiple cellular processes including angiogenesis and tumor growth. It activates multiple signal transduction pathways including Src/focal adhesion kinase (FAK) pathways, STAT 3 pathways, PI3K/Akt pathway and the Ras/MEK pathway (37). AMG 102, a human IgG2 monoclonal antibody directed against the human HGF is currently in a phase I/II trial in combination with platinum based chemotherapy in first line setting for ES-SCLC (NCT00791154). There are several other HGF/c-MET inhibitors/antagonists that are in preclinical studies.
Apoptosis promoters
26S ubiquitin-proteosome complex inhibition
Bortezomib is an inhibitor of the 26S ubiquitin-proteosome complex and was noted in preclinical studies to inhibit the growth of SCLC by inhibiting the antiapoptotic Bcl-2 signaling pathway. Although one phase II trial evaluating single agent Bortezomib failed to show its efficacy in SCLC (38), its combination with topotecan which is an apoptotic trigger is being evaluated in phase I trials in advanced solid tumors (NCT00388089, NCT00541359).
Agents targeting several other pathways such as Polo-like kinase 1 and histone deacetylase have shown promising results in preclinical models, but the results of clinical trials so far have not been encouraging.
BH3 mimetics with or without TORC1/2 inhibition
BH3 mimetic ABT-263 induces apoptosis in a number of cancer cell lines. A high expression of proapoptotic gene Bcl2-interactive mediator of cell death (BIM) predicts greater sensitivity to ABT-263, and SCLC cell lines inherently possessing greater BIM transcript levels, are more sensitive to ABT-263. However, a high expression of antiapoptotic myeloid cell leukemia 1 (MCL-1) confers resistance to ABT-263 by sequestering BIM and preventing BIM mediated apoptosis. ABT-263 in combination with TORC1/2 inhibitor AZD8055 which reduces MCL-1 protein level has been shown to induce marked apoptosis in vitro as well as in TP53;Rb1 deletion mice models of SCLC compared to either drug alone (39). This combination could serve as a potential therapeutic strategy and is in the early phase of development.
Immunotherapy
Recent data in SCLC have shown improvement in PFS and 2-year OS with chest radiotherapy to the primary tumor in the setting of ES-disease following first line platinum based chemotherapy (40) which is akin to the advantage of nephrectomy in the setting of metastatic renal cell cancer (41). One of the postulated mechanisms for such an advantage is that the primary tumor may act as immunologic sink, thereby diverting circulating antibodies and lymphocytes away from the sites of distant metastasis (42). Another theory suggests that the bulk of the primary tumor may suppress host's antitumor response through potentiating tolerance to the mass (41). A subset of SCLCs elicit CD4 dependent antibodies and CD8 T-cell responses against neuronal antigens expressed by the tumor leading to immune mediated paraneoplastic syndromes. SCLC patients with neurologic paraneoplastic syndromes and anti-Hu have an improved response to therapy and prolonged survival. These observations suggest that targeting SCLC immunologically could be successful (43-45).
Vaccines
GD3 is a cell membrane ganglioside that has been shown to be overexpressed in approximately 60% of SCLC tumors (46), and has been investigated as a potential vaccine target for SCLC. Bec2, an anti-idiotypic monoclonal antibody that induces antiganglioside GD3 antibodies, was explored in a small phase I/II trial in which LS-SCLC patients achieving a partial or complete response with induction therapy were vaccinated with Bec2/Bacille Calmette-Guerin. Improved survival was noted among those patients who developed anti-GD3 antibodies compared with historical controls (47). However, results from a subsequent randomized, phase III trial in 515 LS-SCLC patients did not confer any benefit of the vaccine in terms of OS or quality of life (48). Only one third of evaluable patients in the vaccine arm developed a humoral response and the survival in this subgroup was not different compared with nonresponders (19.2 versus 13.9 months, respectively; P=0.085) particularly after adjusting for the use of PCI between the two subgroups.
INGN-225, a p53-modified adenovirus-transduced dendritic cell vaccine that transfers wild type p53 gene in cancer cells was evaluated in phase I/II trial and was well tolerated (all toxicities ≤ grade 2). Specific anti-p53 immune response was positive in 18/43 (41.8%) patients, with overall post-INGN-225 response observed in 17/33 (51.5%) and immune response data available in 29 (14 positive, 15 negative). It also appeared to sensitize SCLC to subsequent chemotherapy. Post-INGN-225 response to chemotherapy was observed in 11/14 (78.6%) and 5/15 (33%) patients with positive and negative immune responses, respectively (49).
Interferon (IFN)
IFN-alpha (IFN-α) is a cytokine that stimulates immune response and promotes antigen presentation on tumor cells (50). Two important studies evaluated the role of IFN-alpha in SCLC. The first of these studies (51) enrolled 237 patients with SCLC who responded to induction chemoradiotherapy who were then randomized to receive maintenance therapy with natural IFN-α, maintenance chemotherapy (cyclophosphamide, adriamycin, and cisplatin), and a control group receiving no maintenance treatment. Median survival in this phase III trial was 11 months in the two maintenance therapy groups and 10 months in the control group. However, there was a significant difference in long-term survival for patients with LS-SCLC, in favor of IFN-α maintenance therapy (P=0.04). Follow-up at 5-year found that 10% of patients in the IFN-α group survived compared with only 2% in the cyclophosphamide, adriamycin, and cisplatin and control groups. However, all the long-term survivors had good performance status and had achieved complete remission with initial induction chemotherapy. Therefore, these findings do not necessarily confirm the role for IFN-α in SCLC.
A second study (52) investigated the feasibility of maintenance therapy with IFN-α plus retinoic acid after a high-dose combination of chemotherapy and radiotherapy in patients with SCLC. Patients in this multicenter, phase II study who responded to chemotherapy and radiotherapy were randomly assigned to one of three maintenance therapy arms: IFN-α and retinoic acid, trophosphamide, or no maintenance treatment (control). Median survival time among the randomized patients was 17.1 months in the IFN-α plus retinoic acid arm, 12.4 months in the trophosphamide arm, and 13.5 months in the control arm, and did not differ significantly between groups. The 1-year survival rate was higher in the IFN-α plus retinoic acid arm (82%) than in the comparator arms (55-56%), and patients treated with IFN-α plus retinoic acid tended to survive longer after the onset of progressive disease, though these differences did not reach statistical significance. The lack of statistically significant outcomes reported in both studies with IFN-α maintenance therapy have led to its cessation of development as an SCLC treatment.
Moreover, a systematic review by Rossi et al. (53) on pooled data from 3,688 patients with SCLC confirmed the lack of survival benefit with IFN-α maintenance therapy.
Immune checkpoint inhibition
More recently, the role of immune checkpoint inhibition with anti-CTLA 4 antibodies and anti-PD1/PDL1 antibodies in SCLC is also being evaluated, especially in combination with chemotherapy and preliminary results are encouraging. Preclinical studies have demonstrated synergy between CTLA-4 blockade and conventional chemotherapy (54) possibly because of augmented release of tumor antigens induced by cytotoxic chemotherapy and their subsequent presentation to T cells by antigen presenting cells. Another theory suggests that chemotherapeutic agents may distort tumor architecture, enhancing the penetration of immunotherapeutic agents. A recent study did suggest that high mutational burden of melanoma correlated with response to CTLA-4 inhibitors (55). This observation might also be the case for PD checkpoint inhibitors as preliminary efficacy has so far been reported in solid tumors with the highest mutational burden including NSCLC, bladder cancer and melanoma. In addition, preliminary data also suggest that the combination of CTLA4 and PD1 inhibitors (ipilimumab plus nivolumab) might have superior efficacy than either agent alone (56).
Ipilimumab is a fully human anti-CTLA-4 antibody and is currently being investigated in SCLC in combination with chemotherapy. A multicenter, international, randomized, blinded phase II study examined two dosing schedules combining platinum-based chemotherapy (paclitaxel/carboplatin) with ipilimumab 10 mg/kg in 334 patients with ES-SCLC (n=130) or stage IV NSCLC (n=204) (57,58). Ipilimumab was given concurrently with paclitaxel/carboplatin or in a phased schedule (two doses of placebo + paclitaxel/carboplatin, followed by four doses of ipilimumab + paclitaxel/carboplatin); or a control regimen of placebo plus paclitaxel/carboplatin (up to six doses of placebo + paclitaxel/carboplatin). The phased schedule of ipilimumab plus platinum-based chemotherapy, resulted in immune related PFS of 6.4 months compared with 5.3 months in the placebo arm (HR =0.64; 95% CI, 0.40-1.02; P=0.03). The best overall response rate (ORR) (71% versus 53%, respectively) using immune related RECIST criteria and OS (12.9 versus 9.9 months, respectively; HR =0.75; 95% CI, 0.46-1.23; P=0.13) also favored phased ipilimumab + paclitaxel/carboplatin over paclitaxel/carboplatin alone, although the difference in survival was not statistically significant. In this study, ES-SCLC cohort was not fully powered for a formal statistical comparison. Nevertheless, these data suggest that this chemoimmunotherapeutic regimen results in improved clinical benefit in patients with ES-SCLC, and that sequence matters. Adverse events that occurred more frequently in the ipilimumab arms than the placebo arm included pruritus (19-24% versus 5%, respectively), rash (24-36% versus 2%, respectively), and diarrhea (26-33% versus 16%, respectively). Overall, the incidence of treatment-related grade 3/4 adverse events appeared more often in the ipilimumab-containing arms, with 43% in the concurrent ipilimumab arm, 50% in the phased ipilimumab arm, and 30% in the placebo arm.
A randomized, multicenter phase III trial is currently underway to determine whether a phased schedule of ipilimumab plus a chemotherapy regimen of etoposide/platinum (carboplatin or cisplatin) versus etoposide/platinum alone extends OS in ES-SCLC patients. The study is estimated to enroll 912 patients on approximately 227 sites among 34 countries (NCT01450761). In addition, a second phase II trial is currently recruiting patients with ES-SCLC to determine whether the addition of ipilimumab to a chemotherapy regimen of etoposide/carboplatin extends PFS at 1 year in this patient population (NCT01331525).
Nivolumab, a fully human IgG4 anti program death receptor-1 (PD-1) antibody is emerging as a promising agent in cancer therapeutics. Its recent approval in the treatment of squamous cell lung cancer is quite encouraging and forms a ground for its further exploration in SCLC. In a phase I/II study, 128 patients with progressive disease after initial platinum based therapy were treated with either nivolumab alone or in combination with ipilimumab. The primary objective was ORR and secondary objectives were safety, PFS, OS, and biomarker analysis. The interim safety and efficacy data were presented at ASCO 2015 annual meeting. The ORR was 15% in patients treated with nivolumab alone and 20% in patients treated with the combination. In addition, the responses were durable (59).
Pembrolizumab, an anti-PD-1 monoclonal antibody, is also being evaluated in SCLC. Preliminary efficacy data show that pembrolizumab is associated with partial responses in 25% of patients with ES-SCLC which is quite promising (60). A multicohort phase Ib study (KEYNOTE-028) evaluated the role of pembrolizumab in advanced solid tumors with high expression of PD-L1. The data presented at the ASCO 2015 annual meeting demonstrated promising antitumor activity of pembrolizumab in PD-L1+ SCLC patients who progressed on platinum based chemotherapy. Of 16 treated patients, 4 had partial response and 1 had stable disease. Six patients had progressive disease and five had not had response assessment at the time of data analysis. All the responders had ongoing response for more than 16 weeks. Although, nearly half of the patients had drug related adverse event (DRAE), only one of them had grade 3 DRAE indicating that pembrolizumab is generally well tolerated (60). Several other studies are ongoing to evaluate the role of immune checkpoint inhibitors in SCLC in various settings including in the maintenance setting of ES-SCLC and in combination with chemoradiotherapy in LS-SCLC. It is unclear whether these strategies can be employed in SCLC. Perhaps the biggest challenge is the fact that immunotherapy does not act immediately and can in fact be associated with pseudoprogression and increase in the volume of the disease. This can be especially dangerous in SCLC patients with large disease burden at presentation and rapid deterioration. In addition one patient on the nivolumab/ipilimumab study died from myasthenia gravis raising the possibility that PD1 inhibition might lead to worsening of paraneoplastic syndromes associated with SCLC. We should therefore be cautious as we evaluate these agents in SCLC.
Inherent genetic complexity with multiple mechanisms of host immune system evasion and lack of predictive markers of response are major challenges in the development of effective immune therapy for SCLC.
Antibody drug conjugate (ADC)
Therapeutic success of T-DM1 in the treatment of HER-2 positive breast cancer has opened doors to evaluation of ADCs in various other malignancies including SCLC. SC16LD6.5 is an ADC with an antibody directed against SC16 protein expressed on tumor cell surface, conjugated with D6.5—a cytotoxic chemotherapeutic agent (61). A phase I/II study of this ADC as a second or third line agent in patients with SCLC is underway (NCT01901653).
Somatostatin receptor inhibition
The majority of SCLCs (50-100%) express somatostatin receptors (type 1-5) with a subset of SCLCs expressing more than one subtype. Stimulation of these receptors leads to inhibition of angiogenesis and cell growth. SOM230 is a somatostatin receptor antagonist that lowers the levels of IGF which is known to contribute to SCLC proliferation (62,63). The combination of SOM230 (pasireotideLAR) with topotecan is being tested in phase II clinical trial for relapsed or refractory SCLC (NCT01417806).
Novel derivatives of conventional chemotherapeutic agents
The complex genomic landscape of SCLC and the heterogeneity are likely major factors contributing to the observation that chemotherapy is currently the only successful systemic strategy in the treatment of ES-SCLC. Palifosfamide is a functionally active metabolite of ifosfamide without any CNS toxicity or hemorrhagic cystitis, thereby conferring easier administration than ifosfamide as well as avoiding the need for close in-hospital monitoring and administration of mesna. Furthermore, in preclinical studies it was found to be active in ifosfamide resistant xenografts as well. Palifosfamide has been evaluated in a phase II study in combination with doxorubicin in patients with soft tissue sarcoma (PICASSO) with primary end point being PFS. The study was terminated early because of favorable outcomes. However the phase III PICASSO trial was negative with no improvement in outcomes with the addition of palifosfamide to doxorubicin in sarcoma. Palifosfamide was then evaluated in ES-SCLC in the MATISSE Study (multinational adaptive trial investigating SCLC survival endpoints). Patients with ES-SCLC were randomized to receive either carboplatin/etoposide or carboplatin/etoposide + palifosfamide. The primary endpoint was OS and patients were stratified by age, gender and performance status. The addition of palifosfamide did not improve OS in this setting and palifosfamide is no longer being developed in the US (64).
Other novel derivatives of the conventional chemotherapy drugs which are currently being tested in phase II/III clinical trials in relapsed/refractory SCLC include combination of liposomal doxorubicin with ifosfamide, NK012 (new SN-38 incorporating miceller nanoparticle), and aldoxorubicin (albumin binding prodrug allowing administration of higher doses than standard doxorubicin, NCT02200757). These agents are designed to minimize toxicities and improve drug delivery of conventional chemotherapies.
Summary
SCLC is a heterogeneous and genetically complex disease with a very high mortality rate. Current standard of care includes concurrent chemoradiation with cisplatin and etoposide for LS-SCLC and combination of platinum and etoposide or irinotecan for ES-SCLC. Despite recent advances in the understanding of SCLC biology and the novel cancer therapeutics, prognosis of SCLC remains dismal. Thus far, only conventional chemotherapy has been proven to be effective in improving OS in large randomized controlled trials. Several new therapeutic targets and strategies including novel cell signaling pathways, immune pathways, and non-traditional chemotherapeutic agents have shown encouraging results in pre-clinical and early clinical trials, but their utility in clinical practice needs to be determined in randomized trials.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [PubMed]
- Johnson BE, Grayson J, Makuch RW, et al. Ten-year survival of patients with small-cell lung cancer treated with combination chemotherapy with or without irradiation. J Clin Oncol 1990;8:396-401. [PubMed]
- Pleasance ED, Stephens PJ, O’Meara S, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature 2010;463:184-90. [PubMed]
- Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol 2001;28:3-13. [PubMed]
- Mori N, Yokota J, Akiyama T, et al. Variable mutations of the RB gene in small-cell lung carcinoma. Oncogene 1990;5:1713-7. [PubMed]
- Arriola E, Cañadas I, Arumí M, et al. Genetic changes in small cell lung carcinoma. Clin Transl Oncol 2008;10:189-97. [PubMed]
- Yokomizo A, Tindall DJ, Drabkin H, et al. PTEN/MMAC1 mutations identified in small cell, but not in non-small cell lung cancers. Oncogene 1998;17:475-9. [PubMed]
- Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. [PubMed]
- Shibata T, Kokubu A, Tsuta K, et al. Oncogenic mutation of PIK3CA in small cell lung carcinoma: a potential therapeutic target pathway for chemotherapy-resistant lung cancer. Cancer Lett 2009;283:203-11. [PubMed]
- Onuki N, Wistuba II, Travis WD, et al. Genetic changes in the spectrum of neuroendocrine lung tumors. Cancer 1999;85:600-7. [PubMed]
- Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc 2008;83:355-67. [PubMed]
- Peifer M, Fernández-Cuesta L, Sos ML, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet 2012;44:1104-10. [PubMed]
- Wang Z, Sun Y. Targeting p53 for Novel Anticancer Therapy. Transl Oncol 2010;3:1-12. [PubMed]
- Stovold R, Meredith SL, Bryant JL, et al. Neuroendocrine and epithelial phenotypes in small-cell lung cancer: implications for metastasis and survival in patients. Br J Cancer 2013;108:1704-11. [PubMed]
- Patton JF, Spigel DR, Greco FA, et al. Irinotecan (I), carboplatin (C), and radiotherapy (RT) followed by maintenance bevacizumab (B) in the treatment (tx) of limited-stage small cell lung cancer (LS-SCLC): Update of a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol 2006;24:abstr 7085.
- Spigel DR, Hainsworth JD, Farley C, et al. Tracheoesophageal (TE) fistula development in a phase II trial of concurrent chemoradiation (CRT) and bevacizumab (B) in limited-stage small-cell lung cancer (LS-SCLC). J Clin Oncol 2008;26:abstr 7554.
- Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215-22. [PubMed]
- Jalal S, Bedano P, Einhorn L, et al. Paclitaxel plus bevacizumab in patients with chemosensitive relapsed small cell lung cancer: a safety, feasibility, and efficacy study from the Hoosier Oncology Group. J Thorac Oncol 2010;5:2008-11. [PubMed]
- Ready NE, Pang HH, Gu L, et al. Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance). J Clin Oncol 2015;33:1660-5. [PubMed]
- Allen JW, Moon J, Redman M, et al. Southwest Oncology Group S0802: a randomized, phase II trial of weekly topotecan with and without ziv-aflibercept in patients with platinum-treated small-cell lung cancer. J Clin Oncol 2014;32:2463-70. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [PubMed]
- Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2012;2:798-811. [PubMed]
- Cardnell RJ, Feng Y, Diao L, et al. Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res 2013;19:6322-8. [PubMed]
- Wainberg ZA, Rafii S, Ramanathan RK, et al. Safety and antitumor activity of the PARP inhibitor BMN673 in a phase 1 trial recruiting metastatic small-cell lung cancer (SCLC) and germline BRCA-mutation carrier cancer patients. J Clin Oncol 2014;32:abstr 7522.
- Mita M, Gordon M, Rosen L, et al. Phase 1B study of amuvatinib in combination with five standard cancer therapies in adults with advanced solid tumors. Cancer Chemother Pharmacol 2014;74:195-204. [PubMed]
- Byers L, Horn L, Gandhi J, et al. A phase 2 study of Amuvatinib (MP-470), the first RAD51 inhibitor in combination with platinum-etoposide (PE) in refractory or relapsed small cell lung cancer (ESCAPE). Cancer Res 2013;73:abstr 2095.
- Hamilton G, Klameth L, Rath B, et al. Synergism of cyclin-dependent kinase inhibitors with camptothecin derivatives in small cell lung cancer cell lines. Molecules 2014;19:2077-88. [PubMed]
- Augustyn A, Borromeo M, Wang T, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 2014;111:14788-93. [PubMed]
- Justilien V, Fields AP. Molecular pathways: novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells. Clin Cancer Res 2015;21:505-13. [PubMed]
- Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med 2011;17:1504-8. [PubMed]
- Rodon J, Tawbi HA, Thomas AL, et al. A phase I, multicenter, open-label, first-in-human, dose-escalation study of the oral smoothened inhibitor Sonidegib (LDE225) in patients with advanced solid tumors. Clin Cancer Res 2014;20:1900-9. [PubMed]
- Belani CP, Dahlberg SE, Rudin CM, et al. Three-arm randomized phase II study of cisplatin and etoposide (CE) versus CE with either vismodegib (V) or cixutumumab (Cx) for patients with extensive stage-small cell lung cancer (ES-SCLC) (ECOG 1508). J Clin Oncol 2013;31:abstr 7508.
- Pannuti A, Foreman K, Rizzo P, et al. Targeting Notch to target cancer stem cells. Clin Cancer Res 2010;16:3141-52. [PubMed]
- Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther 2014;141:140-9. [PubMed]
- Pietanza MC. Final results of phase Ib of tarextumab (TRXT, OMP-59R5, anti-Notch2/3) in combination with etoposide and platinum (EP) in patients (pts) with untreated extensive-stage small-cell lung cancer (ED-SCLC). J Clin Oncol 2015;33:abstr 7508.
- Rudin CM, Durinck S, Stawiski EW, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012;44:1111-6. [PubMed]
- You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep 2008;41:833-9. [PubMed]
- Lara PN Jr, Chansky K, Davies AM, et al. Bortezomib (PS-341) in relapsed or refractory extensive stage small cell lung cancer: a Southwest Oncology Group phase II trial (S0327). J Thorac Oncol 2006;1:996-1001. [PubMed]
- Faber AC, Farago AF, Costa C, et al. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc Natl Acad Sci U S A 2015;112:E1288-96. [PubMed]
- Slotman BJ, van Tinteren H, Praag JO, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet 2015;385:36-42. [PubMed]
- Freed SZ. Nephrectomy for renal cell carcinoma with metastases. Urology 1977;9:613-6. [PubMed]
- Robertson CN, Linehan WM, Pass HI, et al. Preparative cytoreductive surgery in patients with metastatic renal cell carcinoma treated with adoptive immunotherapy with interleukin-2 or interleukin-2 plus lymphokine activated killer cells. J Urol 1990;144:614-7; discussion 617-8. [PubMed]
- Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003;349:1543-54. [PubMed]
- Brahmer JR, Pardoll DM. Immune checkpoint inhibitors: making immunotherapy a reality for the treatment of lung cancer. Cancer Immunol Res 2013;1:85-91. [PubMed]
- Roberts WK, Deluca IJ, Thomas A, et al. Patients with lung cancer and paraneoplastic Hu syndrome harbor HuD-specific type 2 CD8+ T cells. J Clin Invest 2009;119:2042-51. [PubMed]
- Brezicka T, Bergman B, Olling S, et al. Reactivity of monoclonal antibodies with ganglioside antigens in human small cell lung cancer tissues. Lung Cancer 2000;28:29-36. [PubMed]
- Grant SC, Kris MG, Houghton AN, et al. Long survival of patients with small cell lung cancer after adjuvant treatment with the anti-idiotypic antibody BEC2 plus Bacillus Calmette-Guérin. Clin Cancer Res 1999;5:1319-23. [PubMed]
- Giaccone G, Debruyne C, Felip E, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study). J Clin Oncol 2005;23:6854-64. [PubMed]
- Chiappori AA, Soliman H, Janssen WE, et al. INGN-225: a dendritic cell-based p53 vaccine (Ad.p53-DC) in small cell lung cancer: observed association between immune response and enhanced chemotherapy effect. Expert Opin Biol Ther 2010;10:983-91. [PubMed]
- Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol 2013;8:587-98. [PubMed]
- Mattson K, Niiranen A, Ruotsalainen T, et al. Interferon maintenance therapy for small cell lung cancer: improvement in long-term survival. J Interferon Cytokine Res 1997;17:103-5. [PubMed]
- Ruotsalainen T, Halme M, Isokangas OP, et al. Interferon-alpha and 13-cis-retinoic acid as maintenance therapy after high-dose combination chemotherapy with growth factor support for small cell lung cancer--a feasibility study. Anticancer Drugs 2000;11:101-8. [PubMed]
- Rossi A, Garassino MC, Cinquini M, et al. Maintenance or consolidation therapy in small-cell lung cancer: a systematic review and meta-analysis. Lung Cancer 2010;70:119-28. [PubMed]
- Lee F, Jure-Kunkel MN, Salvati ME. Synergistic activity of ixabepilone plus other anticancer agents: preclinical and clinical evidence. Ther Adv Med Oncol 2011;3:11-25. [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371:2189-99. [PubMed]
- Antonia SJ. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol 2015;33:abstr 7503.
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol 2013;24:75-83. [PubMed]
- Antonia SJ, Bendell JC, Taylor MH, et al. Phase I/II study of nivolumab with or without ipilimumab for treatment of recurrent small cell lung cancer (SCLC): CA209-032. J Clin Oncol 2015;33:abstr 7503.
- Ott PA, Fernandez ME, Hiret S, et al. Pembrolizumab (MK-3475) in patients (pts) with extensive-stage small cell lung cancer (SCLC): Preliminary safety and efficacy results from KEYNOTE-028. J Clin Oncol 2015;33:abstr 7502.
- Antibody-drug conjugate SC16LD6.5. Cited 2015 07/03/2015. Available online: http://www.cancer.gov/publications/dictionaries/cancer-drug?cdrid=751419
- Jaques G, Rotsch M, Wegmann C, et al. Production of immunoreactive insulin-like growth factor I and response to exogenous IGF-I in small cell lung cancer cell lines. Exp Cell Res 1988;176:336-43. [PubMed]
- Nakanishi Y, Mulshine JL, Kasprzyk PG, et al. Insulin-like growth factor-I can mediate autocrine proliferation of human small cell lung cancer cell lines in vitro. J Clin Invest 1988;82:354-9. [PubMed]
- Jalal SI. Results from a randomized study of carboplatin and etoposide (CE) with or without palifosfamide (Pa) in extensive stage small cell lung cancer (ES-SCLC): The MATISSE study. J Clin Oncol 2015;33:abstr 7504.


