
Emerging and multifaceted role of neutrophils in lung cancer
Lung cancer
Lung cancer is regarded as a highly preventable disease, where the leading risk factor, cigarette smoking, accounts for approximately 80% of diagnoses. Cigarette smoke contains multiple classes of recognized carcinogens including benzo(a)pyrenes, polycyclic aromatic hydrocarbons, and tobacco specific nitrosamines (1,2). Collectively, these compounds exert their genotoxic effects by forming DNA adducts and by generating reactive oxygen species (ROS) that cause mutations in critical growth control genes, such as K-Ras and TP53 (3-6). In addition, chronic smoking causes a state of chronic injury and inflammation in the airways, and inflammation has been identified as an enabling characteristic for tumour development (7). Like lung cancer, chronic obstructive pulmonary disease (COPD) is a common debilitating disease caused by consumption of cigarette smoke and other noxious air pollutants. COPD is currently the 4th leading cause of death globally and is characterized as a chronic inflammatory condition due to the progressive nature of mucosal inflammation and destruction that manifests into rapid lung function decline (8). It is well established that excessive airway neutrophil recruitment and activation contributes to tissue destruction in COPD. COPD patients are also at an increased risk of developing lung cancer and this risk is not solely driven by smoke exposure. In this review, we will focus on the emerging evidence to suggest that neutrophils actively contribute to the tumour microenvironment (TME).
Neutrophil recruitment and activation
Neutrophils are the most abundant circulating leukocytes in human blood and are considered the first line of innate immune defence. Under homeostatic conditions, neutrophils are generated from the bone marrow at a rate of 1011 cells per day. From the bone marrow, they are released and remain in circulation until recruited to tissues where they perform a suite of specialised functions, which include phagocytosis, degranulation, oxidative burst, and the release of neutrophil extracellular traps (NETs) (9). At the end of their lifespan they are cleared away by tissue-resident macrophages via phagocytosis (10). In the blood, these cells have a very shot half-life of 6–8 hrs, and are subsequently destroyed by the spleen, liver, and the bone marrow itself (11). Despite this large daily turnover, neutrophil numbers remain consistent through a careful balance of granulopoiesis, bone marrow storage and release, and migration into tissues (12).
Neutrophils are produced in the bone marrow from hematopoietic stem cells (HSCs). These cells then differentiate into multipotent progenitors (MPP), next into lymphoid primed multipotent progenitors (LMPPs) and subsequently into granulocyte-monocyte myeloid progenitors (GMPs) (13). Granulocyte-colony stimulating factor (G-CSF) is the master regulator of neutrophil generation and differentiation and binds to a high-affinity receptor, G-CSFR, which is expressed by developing neutrophils throughout the myeloid lineage. Under the control of G-CSF, GMPs commit to neutrophil formation and the hallmark granules are variably formed at specific steps during myeloid differentiation (14). Thus, expression of these granules is a representation of neutrophil maturity. During differentiation, primary granules are formed first, at the myeloblast to promyelocyte stage. These granules contain large amounts of myeloperoxidase (MPO) and neutrophil elastase (NE). Next, secondary granules form at the myelocyte and metamyelocyte stages. These granules are rich in lactoferrin, primarily involved in host defense against microbial infection. Tertiary granules rich in gelatinase then form in band and segmented neutrophils, and finally, secretory vesicles arise in mature cells, which contain plasma proteins and membrane-associated receptors (14,15).
As neutrophils mature, they downregulate the expression of various receptors including mast/stem cell growth factor receptor (KIT), integrin alpha 4 (VLA4) and C-X-C chemokine receptor 4 (CXCR4), while upregulating C-X-C chemokine receptor 2 (CXCR2) and toll-like receptor 4 (TLR4) (14). Once neutrophils mature, they can leave the bone marrow and enter circulation, where their release is tightly regulated, since under normal homeostatic conditions only 1–2% of all neutrophils in the body are found in the blood (16). Under steady-state granulopoiesis, ligands for KIT, VLA4 and CXCR4 are produced in the bone marrow to retain neutrophils, while ligands for CXCR2, such as CXCL1, CXCL2, CXCL5 and CXCL8/IL-8 are produced externally from endothelial cells and megakaryocytes to induce neutrophil mobilization when required (14,16,17). Neutrophil mobilization can also be regulated by a cytokine network that involves interleukin 23 (IL-23), that is produced by phagocytic macrophages and dendritic cells (DCs), and interleukin 17 (IL-17), that is produced by T lymphocytes (T cells). IL-23 drives and maintains the differentiation of T helper 17 (Th17) that produce IL-17, which promotes granulopoiesis and the release of neutrophils from the bone marrow via upregulation of G-CSF. This process is self-limiting as the phagocytosis of apoptotic neutrophils triggers an anti-inflammatory response characterised by a reduction in IL-23 by tissue-resident macrophages. Subsequently, reduced IL-23 levels lead to a decrease in IL-17 levels and to less G-CSF production, which is central to the maintenance of steady-state neutrophil release from the bone marrow (18). Additionally, it has been shown that CXCR4 expression can be spontaneously upregulated in neutrophils cultured for 4 hours, where re-expression of CXCR4 is thought to encourage aging neutrophils to return to the bone marrow for clearance (19).
In contrast, neutrophils at are found to reside in a range of maturation stages in the blood and tissue of cancer patients, where the impact of neutrophil maturity on cancer progression and therapy remains largely unknown (20). It is not clear how tumours induce neutrophil trafficking, however it is suggested that mobilization occurs via tumour produced cytokines, where G-CSF levels and CXCR2 ligands are elevated in patients with NSCLC (21,22). Additionally, the recruitment and activation of neutrophils to solid tumours is said to be controlled by conditions within the TME, such as hypoxia, nutrient starvation, and necrosis leading to the release of damage associated molecular patterns (DAMPs) (23). Maturation status is also associated with neutrophil lifespan. As a result neutrophils are found to survive approximately 2.5-fold longer in cancer compared to neutrophils produced under normal homeostatic conditions (14), where these neutrophils are shown to regulate inflammation and the immune system by modulating the activity of neighboring cells and thus actively take part in cancer progression (24).
Chronic inflammation may be pathogenic in lung cancer
The connection between inflammation and cancer development is an established concept that stems from the comparison of the tumour with a wound that does not repair, where tumours harness the wound healing response to induce cancer cell maintenance and growth (25). The tumor microenvironment (TME) consists of the extracellular matrix (ECM), the blood and lymphatic vascular network, and key cellular players such as fibroblasts and a dynamic network of immune and inflammatory cells that will be influenced by underlying conditions such as COPD. While the innate and adaptive immune systems typically operate in a transient manner under physiological stresses such as respiratory infections, chronic inflammation consisting of T cells, natural killer cells (NK cells), macrophages, and neutrophils can persist in the lung cancer landscape. Specialized leukocyte populations, such NK cells and cytotoxic T cells (CTLs) are recognized for their potent antitumor activity, whereas macrophages and neutrophils are shown to exhibit both anti- and pro-tumorigenic behavior (26). Therefore, the interaction between malignant cells and immune cell subsets of the TME can ultimately determine whether the primary tumour is eradicated or metastasized. It has become evident that the TME can shape therapeutic responses and resistance, emphasizing the need to develop new therapeutic strategies targeting specific TME immune components (27). This is best exemplified by the success of immune checkpoint inhibitors (ICI) in the clinic, which facilitates the recognition and death of malignant cells by reactivating T cells (28). In addition to the adaptive response the innate response is also altered in lung cancer where an increase in tumour associated macrophages (TAMs) and tumour associated neutrophils (TANs) are observed (14,26). The interaction between innate and adaptive cells will clearly influence cytotoxic recognition and killing of cancer cells, and emerging findings are revealing surprising and pleotropic roles for neutrophils in the TME.
Like lung cancer, COPD is a common debilitating disease caused by consumption of cigarette smoke and other noxious air pollutants. COPD is currently the 4th leading cause of death globally and is characterized as a chronic inflammatory condition due to the progressive nature of mucosal inflammation and destruction that manifests into rapid lung function decline (8). COPD patients are 4–6-fold more likely to develop lung cancer, which is unsurprising given the shared risk factor of chronic smoking. However, it has become increasingly apparent that smoking alone does not account for why COPD patients are much more likely to develop lung cancer. A strong linear relationship between increasing severity of airflow limitation in COPD and risk of lung cancer was reported to be independent of smoking (29,30). The degree of airway inflammation is also known to increase with the severity of COPD, whereby the infiltration and accumulation of inflammatory cells into the lung, including neutrophils, monocytes, macrophages, and lymphocytes occurs. The neutrophil is particularly associated with disease pathogenesis in COPD, with neutrophilia being a characteristic feature of COPD severity (31,32). There is also direct evidence for dysfunctional neutrophil migration, degranulation, protease release and ROS production in COPD patients (32-34). The persistent infiltration of macrophages and neutrophils in response to cigarette smoking is dependent on IL-17A signaling, as depletion of this cytokine prevents inflammation (35). Another important feature of COPD is that as severity increases, so does risk of recurrent infection-driven exacerbations. Acute exacerbations of COPD (AECOPD) are characterized by a marked increase in local and systemic inflammation that includes neutrophilic inflammation. AECOPD are also predictive of lung cancer, independently of lung function, age or pack years smoked (36). Current pharmacological therapies including the combination of inhaled corticosteroids and bronchodilators do not slow the progression of lung function decline, but may help to reduce the rate of exacerbations in severe patients that frequently experience a decline in their condition.
It is documented that the long-term use of inhaled anti-inflammatory corticosteroids (ICS) in COPD patients is associated with a reduced risk of lung cancer, which is the most common cause of death among COPD sufferers. In a cohort of COPD patients with no lung cancer history, Parimon et al. demonstrated that COPD patients on high doses of ICS (1,200 µg/day) were 2-fold less likely to develop lung cancer (37). A similar outcome was also observed in a large cohort of South Korean participants. Eligibility was defined as patients aged 30–89, lacking a lung cancer history, and newly diagnosed with COPD with an initiation of ICS after diagnosis. Paralleling Parimon’s study, Lee et al. showed that participants in a high cumulative ICS dose group had a 2-fold reduced risk of lung cancer compared to those in the low-dose group (38). However, these findings were markedly more prominent in men. Equally, due to the limited number of women enrolled in Parimon’s study, a sex-specific effect remains inconclusive, noting that both study outcomes may not be generalizable to women with COPD. Nonetheless, the observation that patients with COPD demonstrated a reduction in lung cancer incidence following ICS treatment supports the concept that inflammation may be pathogenic by promoting lung tumour development.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly prescribed worldwide and the sheer number of people on long-term NSAIDs such as daily use of low-dose aspirin, typically prescribed for secondary prevention of myocardial infarction and stroke, provide compelling epidemiological data for the role of inflammation in cancer. Randomized controlled trials have reliably identified a chemopreventive benefit of aspirin in colorectal cancers in a duration-dependent manner (39), whereas the effect of aspirin in lung cancer prevention remains inconclusive with either negative findings or modest benefits (40). However, studies which have stratified lung cancer incidence by histological subtype have shown benefit that appears to be restricted to the most common subtype of lung cancer, adenocarcinoma (ADC) (39). It is well established that aspirin’s anti-inflammatory actions are elicited via modulation of the inducible cyclooxygenase-2 (COX-2) enzyme, inhibiting prostaglandin formation. However, it also non-selectively targets the constitutive cyclooxygenase-1 (COX-1) isoform, associated with homeostatic maintenance and gastrointestinal injury when repressed (41). Further clinical trials are needed to make a more definitive recommendation that addresses the risk-benefit ratio for the recommendation of daily low-dose aspirin in the prevention of lung cancer.
Plasticity of neutrophils in the TME
The historic view of neutrophils has changed considerably from a homogenous population of terminally differentiated cells with a repertoire of antimicrobial effector functions to cells with a large phenotypic heterogeneity and functional versatility. It has become evident that the microenvironment in different tissues can stimulate neutrophils to obtain specialised functions. In the lung, many neutrophils accumulate to the vascular lumen and in the interstitial space where they regain CXCR4 expression (42). In the spleen, neutrophils locate to the marginal zone to produce cytokines that induce antibody production in marginal B lymphocytes (43). Additional subpopulations of neutrophils that can migrate to the lymph nodes due to the expression of C-C chemokine receptor type 7 (CCR7), lymphocyte function-associated antigen 1 (LFA-1) and CXCR4 are found to mediate T cell activation (44,45). Furthermore, a proangiogenic neutrophil subtype in the tissues exists (46). In pathological conditions such as inflammation and cancer various neutrophil phenotypes can be found (47-49). This is particularly evident in cancer where neutrophil phenotype is shown to change with tumour progression and several neutrophil subpopulations with different characteristics of maturity, tumour cytotoxicity and immune suppression result (50,51).
An increase in the circulation of immature myeloid cells is observed in many types of cancers and is believed to occur due to perturbed myelopoiesis in tumour-bearing hosts. These cells, which are derived from the bone marrow like neutrophils, are termed myeloid derived suppressor cells (MDSCs). MDSCs can be divided into two distinct groups of cells termed granulocytic- or polymorphonuclear-MDSCs (G-MDSCs or PMN-MDSCs), which are phenotypically and morphologically similar to neutrophils, and monocytic-MDSCs (M-MDSCs), which are phenotypically and morphologically similar to monocytes (52). Currently, there appears to be no universal cell surface markers capable of accurately distinguishing neutrophils from PMN-MDSCs (53). However, high expression of Lectin-type oxidized LDL receptor 1 (LOX-1) was found to be exclusive to PMN-MDSCs in various cancers, including NSCLC (54). Low-density neutrophils (LDNs) are another circulating neutrophil subset that requires further characterization in lung cancer. This subset was first identified when neutrophils co-segregated with mononuclear cells after density-gradient isolation in systemic lupus erythematosus and rheumatoid arthritis patients (55). While their role in driving lung cancer is largely unknown, elevated LDN levels have been detected in patients with advanced ADC and correlate with poorer prognosis (56).
Within the TME, TANs could be considered as one of the emerging targets for multiple cancer types, where therapeutic intervention can be meditated by inhibiting neutrophil trafficking and activation (14). It has been demonstrated that cells of the myeloid lineage are abundant within the NSCLC TME, accounting for approximately 50% of tumor-infiltrating CD45+ cells, where neutrophils alone comprise 20% of the CD45+ population (53). This places neutrophils as a dominant immune cell type present in the tumor and stroma of NSCLC, with greater neutrophil presence in the smoking-related squamous cell carcinoma (SCC) subtype and is consistent with persistent neutrophilic inflammation observed in COPD. There is a positive correlation between neutrophil numbers and tumor growth, indicating that TANs may serve as an important diagnostic and prognostic indicator in lung cancer (57). Pre-clinical studies have demonstrated the importance of neutrophils in tumour progression, where TANs initially display anti-tumour actions, and appear to transition towards a pro-tumorigenic phenotype at later stages (58).
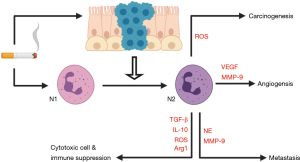
One of the major distinctions between TAN populations in cancer relates to the N1 and N2 classification. While these subsets are specific to neutrophil populations observed in the TME, the N1 and N2 naming convention has also been utilised to describe the polarization of pro-inflammatory (N1) to anti-inflammatory (N2) neutrophils isolated from infarcted left ventricles in mice (59). N1 TANs can promote anti-tumour effector functions with increased tumor cytotoxicity, high expression of NETs, increased secretion of immuno-activating cytokines, and low expression of arginase (23). At the early stages of tumour development N1 TANs predominate, however as tumours progress N2 neutrophils and/or PMN-MDSCs are shown to accumulate in favor of the N1 phenotype, as shown in Figure 1. The mechanism leading to this switch in phenotype, also termed ‘neutrophil polarization’, remains largely unknown due to the uncertainty of whether mature neutrophils in circulation can be reprogrammed by external stimuli, or whether defined phenotypes are programmed in the bone marrow. Evidence suggests that neutrophils are very plastic cells and subsequently the various subtypes are more likely to be acquired in the tissues, where it has been demonstrated that neutrophil polarization can be regulated by the balance of the cytokines transforming growth factor beta (TGF-β) and interferon beta (IFN-β) in the TME (60,61). Contrasting the function of N1 neutrophils, the N2 neutrophils display pro-tumorigenic activity, heightened MMP-9 expression, and high arginase expression. When N2 cells accumulate, increased angiogenesis, tumor cell proliferation, ECM remodelling, and inhibition of the anti-tumoral immune response are observed (61,62). These cancer promoting processes are facilitated by the secretion of pro-inflammatory, proliferative, pro-angiogenic and immunoregulatory cytokines such as tumor necrosis factor alpha (TNFα), interleukin 6 (IL-6), epidermal growth factor (EGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), interleukin 10 (IL-10) and TGF-β, which can profoundly shape the tumour landscape (63). Neutrophils can also modify the TME via the secretion of a range of proteolytic enzymes.
The role of neutrophil-derived proteolytic enzymes in NSCLC
NE is a major contributor to the development of emphysema in COPD and is reported to exert pro-tumorigenic actions in a range of malignancies, facilitating both primary tumor growth and secondary organ metastasis. Secreted by neutrophils during inflammation, NE is released into the TME through degranulation and during neutrophil extracellular trap formation (NETosis), which destroys pathogens and host tissue alike (64). In lung cancer patients, NE levels are elevated and positively correlate with disease progression. Interestingly, it has been shown that in both bronchoalveolar lavage fluid (BALF) and serum, NE activity is up to 5-fold higher in individuals with lung cancer compared to those with COPD, highlighting a heterogeneity in its expression in different pathologies (65). NE can also regulate the activity of several members of the matrix metalloproteinase (MMP) family, including MMP-9. This is achieved through direct action on the pro-enzymes as well as inactivation of MMP regulators, such as tissue inhibitor of metalloproteinase-3 (TIMP-3). The MMP-9 to TIMP-3 ratio is markedly elevated in NSCLC, and serves as a diagnostic tool for the identification of malignant cells (66). Whilst MMP-9 is typically secreted from a range of inflammatory cells, in lung tumor tissue acquired from NSCLC patients, TANs have been identified as the primary source of this proteinase (67). Several studies have outlined that high MMP-9 expression correlates positively with aggressiveness of malignant cells in solid tumors. This is largely linked to their inherent ability to degrade ECM components such as elastin, collagens, and fibrinogen. This facilitates both the intravasation of cancer cells into the bloodstream and the sequestering of matrix-bound cytokine receptors, adhesion molecules and angiogenic factors, where MMP-9 mediated sequestering of VEGF from the ECM is shown to be a principle driver of the ‘angiogenic switch’, which leads to neovascularization and tumor expansion (62). Unfortunately, drugs developed to inhibit MMPs in NSCLC have failed to improve survival rates and produced unforeseen side effects in patients (68,69). In these clinical trials the small-molecule metalloproteinase inhibitors (MPIs) were administered to patients with an advanced disease, however, evidence suggests that MMP inhibition is more important in early stages of tumorigenesis (70). Additionally, some MMPs can display anti-tumor effects related to their ability to degrade plasminogen, collagen XVIII, and collagen IV to produce natural angiogenic inhibitors, such as angiostatin, endostatin and tumstatin (71). Therefore, any broad spectrum MPIs may mitigate any potential benefit in blocking proteases such as MMP-9.
The role of neutrophils in T cell lymphocyte suppression
The neutrophil to lymphocyte ratio (NLR) has gained increased attention as a biomarker across different disease settings, where the NLR serves as a non-invasive and simple test of systemic inflammation. The NLR can also be used as an independent prognostic factor in a variety of cancers, including lung cancer, where an elevated NLR has been associated with poor outcomes in NSCLC patients (57,72). Subsequently, the NLR may be utilised for risk stratification of patients and further exploited to guide treatment decisions, where a reduction in NLR may infer positive response to therapy. However, due to the variability in neutrophil counts between healthy individuals and transient fluctuations, the clinical application of the NLR may require longitudinal repeated measurements (14). The specific molecular mechanisms that influence the NLR in cancer remains incompletely understood. However, the imbalance between neutrophils and T cells in cancer may result because of neutrophil-mediated T cell suppression, which can occur by direct and indirect mechanisms. The immunosuppressive nature of distinct neutrophil phenotypes is an emerging concept where TANs modulate adaptive cytotoxic lymphocyte immune responses in the TME.
Elevated levels of ROS have also been detected in most cancers, where markers of DNA damage and oxidative stress are upregulated in lung cancer (73). It is well established that neutrophils are a main source of ROS in the TME, where ROS generation is governed by the conversion of molecular oxygen (O2) to superoxide (O2−) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (74). Superoxide can be further modified to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). Although imperative for host defence mechanisms, high concentrations of ROS are known to promote various aspects of tumor progression, including inflammation, cell proliferation and survival. Nuclear factor-κB (NF-κB) is a transcription factor that plays a crucial role in these processes. In tumour tissue with elevated NF-κB activity, pro-inflammatory cytokines accumulate (75). ROS is known to stimulate NF-κB activity, thus it can drive the malignant transformation of normal cells and support the pro-tumorigenic microenvironment. Although recent studies with animal models and cell culture systems have established the links between NF-κB and lung carcinogenesis, the clinical value of blocking NF-κB in NSCLC is still controversial (76). Of growing attention is the involvement of ROS in the suppression of T cell responses. The activation of T cells is dependent on the presence of the T cell receptor (TCR) on the cell membrane. The TCR is regulated by continuous internalization and recycling of the receptor chain, in which expression of the TCR-ζ is rate limiting but vital for the stabilization of the TCR, and for signal transduction. H2O2 has been shown to downregulate TCR-ζ chain expression, and thereby suppress T cell activation (77). Additionally, neutrophil generated H2O2 has also been shown to suppress the metabolic switch from mitochondrial respiration to aerobic glycolysis in activated T cells that is required for clonal T cell expansion and cytokine production (78).
Neutrophils may also suppress cytotoxic cell function through the production of the type 2 cytokine IL-10. IL-10 mRNA expression levels in lung tumour biopsies is elevated across all NSCLC histological subtypes when compared to normal lung tissue (79). IL-10 displays a broad array of immune functions, including inhibition of T cell proliferation, type-1 cytokine production, antigen presentation, and lymphokine-activated killer cell cytotoxicity (79-82). More recently, it has been shown that the acute phase protein serum amyloid A (SAA) is implicated in the plasticity of neutrophils, where this immunomodulatory mediator can stimulate the differentiation of IL-10 secreting neutrophils via N-formyl peptide receptor 2 (FPR2) signalling (83). SAA is a potent neutrophil chemokine that can promote neutrophil trafficking into the lungs (84,85). Of notable interest, a positive association between the number of neutrophils and the expression of SAA in peripheral lung carcinoma resection samples has been observed (84). Furthermore, SAA serum and plasma levels are shown to be significantly higher in lung cancer patients (86). Taken-together, these studies support that elevated SAA levels in the TME or systemically may contribute to cancer pathogenesis. The concept that IL-10 secreting neutrophils inhibit CD8+ T cell cytotoxic function is supported by the finding that tumor specific CD8+ T cell proliferation is inhibited in mixed leukocyte cultures compared to peripheral blood mononuclear cell (PBMC) cultures (82). In melanoma patients, neutrophil populations were expanded in the peripheral blood of a large proportion of subjects. Unlike the neutrophils from the healthy donors, constitutively synthesised IL-10 was observed from the neutrophils of melanoma patients and blocking the IL-10 receptor restored CD8+ T cell proliferation. Moreover, melanoma associated neutrophils demonstrated enhanced expression of interleukin 8 (IL-8) and arginase-1 (Arg1), where IL-8 is a classic neutrophil chemokine, and Arg1 suppresses T cell function in the TME due to the depletion of the amino acid L-arginine typically required for T cell proliferation. Expression of both IL-8 and Arg-1 by neutrophils is demonstrated to correlate with poor prognosis in NSCLC (82,87-91).
TGF-β is another key cytokine that has been investigated for its pathogenic role in lung cancer. While TGF-β is secreted from neutrophils, the source of this cytokine in tumours varies but includes both the malignant cells and cells of the tumor stroma where it plays a paradoxical role in cancer. Like neutrophils, it is generally accepted that TGF-β is primarily a tumor suppressor in premalignant cells but functions as a promotor of metastasis at later stages of cancer progression, highlighting both anti- and pro-tumorigenic functions (92). While therapeutic strategies against TGF-β signaling using neutralizing antibodies and small molecular inhibitors have been developed, the mechanism underlying the switch in function from tumour suppressor to tumour promotor remains unclear, and thus presents great challenges for TGF-β-targeted cancer therapy (93). TGF-β presents itself as an important therapeutic target due to the multitude of functions it can regulate in the TME. In particular, TGF-β is shown to skew the polarization of neutrophils to the N2 pro-tumorigenic phenotype, where TGF-β inhibition in animal flank tumor models favored the accumulation of N1 TANs with anti-tumor activity (60). It has been documented that TGF-β levels correlate with tumor progression and prognosis in patients with NSCLC, where higher TGF-β levels are associated with lymph node metastasis (94,95). This may be due to its ability to stimulate angiogenesis and epithelial to mesenchymal transition (EMT), both of which are core to tumour spread and survival. While TGF-β is recognized as a potent inducer of EMT in NSCLC, how it stimulates this process is not fully understood (96). However, it is likely that metastasis of the primary tumor is aided by the interplay between neutrophil secreted MMP-9 and TGF-β in the TME. Although TGF-β is produced by various cell types in the TME, proteolytic activation by MMPs, chiefly MMP-9, is required to exert its cellular functions. This is due to the presence of a latency associated peptide (LAP) bound to TGF-β in its inactive state (97). Moreover, TGF-β in its active form can enhance recruitment of myeloid cells, and upregulate the expression, secretion, and activation of MMPs in both cancer cells and cells of the stroma, while downregulating the expression of TIMPs in the TME (97). Together, these actions foster a bidirectional regulatory loop that can lead to negative patient outcomes.
TGF-β can also negatively impact tumour progression via functioning as an immune suppressor of DCs, which are critical for host immunity by bridging innate and adaptive immunity. DCs infiltrate the TME in response to tumor-derived chemokines, however can be repressed by TGF-β, which inhibits production of interleukin-12 (IL-12) by DCs, a critical cytokine that normally stimulates CD4+ and CD8+ T cell function and activation. Additionally, TGF-β promotes immune suppression by favoring regulatory T cell (Treg) expansion through the induction of the Treg signature transcription factor FOXP3, where Tregs suppress cytotoxic lymphocyte functions (98). Moreover, TGF-β can induce the differentiation of a subset of CD4+ T cells known as Th17 cells. IL-17 is the major effector cytokine derived from Th17 cells (35), and is an inflammatory cytokine that induces the production of IL-6 and G-CSF from tumour and stromal cells, thus promoting the activation and recruitment of neutrophils to the TME (99). Notably, IL-17 is detectable in several tumour types, including lung tumours, where high mRNA transcript levels are associated with resistance to VEGF inhibitors and poor patient outcomes (100). Supporting IL-17’s role in tumor pathogenesis, a significant reduction in tumour growth has been observed following administration of a neutralizing anti-IL-17 antibody in a murine model of lung ADC (101). More specifically, IL-17 was shown to promote lung tumour growth and metastasis via several mechanisms, which include supporting tumour vascularization and EMT (101-103).
K-Ras mutant tumors, which represent approximately 30% of all ADCs (99) have also been shown to display a dependency on IL17-dependent neutrophil trafficking. A bitransgenic mouse model of lung cancer expressing both a conditional IL-17 allele along with a conditional oncogenic form of K-Ras (K-RasG12D) grew tumors more rapidly, resulting in a significant reduction in survival time, compared to mice with a K-Ras mutation only. Additionally, IL-17:K-Ras tumors had significantly more TANs and fewer CD4+ and CD8+ T cells than K-Ras tumors (99). Consequently, resistance to PD-1 immune checkpoint blockade was observed in IL17:K-Ras mice that could be rectified by neutrophil depletion with an anti-Ly6G antibody, which reconstituted T cell activation (99). This suggests that the IL-17-neutrophil axis is pathogenic in the inflammatory lung cancer landscape, and that IL-17 targeting may reduce tumor growth in K-Ras mutant tumours.
Conclusions
The historic perception of neutrophils is that they are short-lived innate immune cells geared towards pathogen clearance with limited capacity to respond to environmental cues. It has now become increasingly clear that neutrophils are an important cellular feature in many solid tumours, including lung cancer. Whilst the relationship between neutrophils and cancer is complex, their prevalence in the lung cancer landscape will be influenced by the systemic and local inflammatory response initiated by inhaled irritants such as tobacco smoke consumption. We now know that as COPD progresses in disease severity, so does the level of neutrophil infiltration and risk for developing lung cancer. NSAIDs also display broad anti-inflammatory actions including inhibition of neutrophil trafficking and degranulation, and it is tempting to speculate whether modest benefits in reducing lung cancer risk are associated with modulating neutrophil function in the TME. Indeed, targeting neutrophils in pre-clinical K-Ras mouse models results in a reduction in tumour burden. Neutrophils play a critical role in various stages of tumour progression, where the TME can influence the emergence of distinct neutrophil phenotypes that produce several key mediators involved in tumour growth and invasiveness. Mediators such as NE, MMP-9, ROS and several cytokines are recognized for their ability to influence the growth of cancer cells. Of emerging interest is how neutrophils utilize these potent tools to engage in crosstalk with cytotoxic cells where TANs can suppress anti-tumoral T cell lymphocyte function. Consistent with this concept, a high NLR is a prognostic marker for poor outcomes in NSCLC patients. In summary, a greater effort should be placed on understanding the biology and plasticity of neutrophils in lung cancer. There is an opportunity to develop selective therapeutic strategies to suppress pathogenic neutrophil phenotypes as an adjuvant therapy to reactivating cytotoxic killing of cancer cells.
Acknowledgments
Funding: This work is supported by funding from NHMRC Australia.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research for the series “Lung cancer and the immune system”. The article has undergone external peer.
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-760
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-760). The series “Lung cancer and the immune system” was commissioned by the editorial office without any funding or sponsorship. DS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Lung Cancer Research from Sep 2019 to Sep 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hecht SS, Hoffmann D. Tobacco-specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 1988;9:875-84. [Crossref] [PubMed]
- Yershova K, Yuan JM, Wang R, et al. Tobacco-specific N-nitrosamines and polycyclic aromatic hydrocarbons in cigarettes smoked by the participants of the Shanghai Cohort Study. Int J Cancer 2016;139:1261-9. [Crossref] [PubMed]
- Ewa B, Danuta MŠ. Polycyclic aromatic hydrocarbons and PAH-related DNA adducts. J Appl Genet 2017;58:321-30. [Crossref] [PubMed]
- Feng Z, Hu W, Chen JX, et al. Preferential DNA damage and poor repair determine ras gene mutational hotspot in human cancer. J Natl Cancer Inst 2002;94:1527-36. [Crossref] [PubMed]
- Liu Z, Muehlbauer KR, Schmeiser HH, et al. p53 mutations in benzo(a)pyrene-exposed human p53 knock-in murine fibroblasts correlate with p53 mutations in human lung tumors. Cancer Res 2005;65:2583-7. [Crossref] [PubMed]
- Pfeifer GP, Denissenko MF, Olivier M, et al. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002;21:7435-51. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Wang Y, Xu J, Meng Y, et al. Role of inflammatory cells in airway remodeling in COPD. Int J Chron Obstruct Pulmon Dis 2018;13:3341-8. [Crossref] [PubMed]
- Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Annu Rev Pathol 2014;9:181-218. [Crossref] [PubMed]
- Poon IK, Lucas CD, Rossi AG, et al. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 2014;14:166-80. [Crossref] [PubMed]
- Summers C, Rankin SM, Condliffe AM, et al. Neutrophil kinetics in health and disease. Trends Immunol 2010;31:318-24. [Crossref] [PubMed]
- von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol 2008;181:5183-8. [Crossref] [PubMed]
- Görgens A, Radtke S, Möllmann M, et al. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Rep 2013;3:1539-52. [Crossref] [PubMed]
- Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016;16:431-46. [Crossref] [PubMed]
- Häger M, Cowland JB, Borregaard N. Neutrophil granules in health and disease. J Intern Med 2010;268:25-34. [PubMed]
- Rosales C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front Physiol 2018;9:113. [Crossref] [PubMed]
- Köhler A, De Filippo K, Hasenberg M, et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 2011;117:4349-57. [Crossref] [PubMed]
- Stark MA, Huo Y, Burcin TL, et al. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity 2005;22:285-94. [Crossref] [PubMed]
- Martin C, Burdon PC, Bridger G, et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 2003;19:583-93. [Crossref] [PubMed]
- Mackey JBG, Coffelt SB, Carlin LM. Neutrophil Maturity in Cancer. Front Immunol 2019;10:1912. [Crossref] [PubMed]
- Bahar B, Acedil Ayc Iota B, Çoşkun U, et al. Granulocyte colony stimulating factor (G-CSF) and macrophage colony stimulating factor (M-CSF) as potential tumor markers in non small cell lung cancer diagnosis. Asian Pac J Cancer Prev 2010;11:709-12. [PubMed]
- Saintigny P, Massarelli E, Lin S, et al. CXCR2 expression in tumor cells is a poor prognostic factor and promotes invasion and metastasis in lung adenocarcinoma. Cancer Res 2013;73:571-82. [Crossref] [PubMed]
- Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev 2016;273:329-43. [Crossref] [PubMed]
- Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood 2014;124:710-9. [Crossref] [PubMed]
- Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res 2015;3:1-11. [Crossref] [PubMed]
- Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev 2018;32:1267-84. [Crossref] [PubMed]
- Hirata E, Sahai E. Tumor Microenvironment and Differential Responses to Therapy. Cold Spring Harb Perspect Med 2017;7:a026781 [Crossref] [PubMed]
- Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol 2017;18:31-41. [Crossref] [PubMed]
- Mannino DM, Aguayo SM, Petty TL, et al. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med 2003;163:1475-80. [Crossref] [PubMed]
- Young RP, Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res 2018;7:347-60. [Crossref] [PubMed]
- Singh D, Edwards L, Tal-Singer R, et al. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE study. Respir Res 2010;11:77. [Crossref] [PubMed]
- Vlahos R, Wark PA, Anderson GP, et al. Glucocorticosteroids differentially regulate MMP-9 and neutrophil elastase in COPD. PLoS One 2012;7:e33277 [Crossref] [PubMed]
- Noguera A, Batle S, Miralles C, et al. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax 2001;56:432. [Crossref] [PubMed]
- Sapey E, Stockley JA, Greenwood H, et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;183:1176-86. [Crossref] [PubMed]
- Bozinovski S, Seow HJ, Chan SP, et al. Innate cellular sources of interleukin-17A regulate macrophage accumulation in cigarette- smoke-induced lung inflammation in mice. Clin Sci (Lond) 2015;129:785-96. [Crossref] [PubMed]
- Carr LL, Jacobson S, Lynch DA, et al. Features of COPD as Predictors of Lung Cancer. Chest 2018;153:1326-35. [Crossref] [PubMed]
- Parimon T, Chien JW, Bryson CL, et al. Inhaled corticosteroids and risk of lung cancer among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:712-9. [Crossref] [PubMed]
- Lee YM, Kim SJ, Lee JH, et al. Inhaled corticosteroids in COPD and the risk of lung cancer. Int J Cancer 2018;143:2311-8. [Crossref] [PubMed]
- Rothwell PM, Fowkes FG, Belch JF, et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 2011;377:31-41. [Crossref] [PubMed]
- Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:47-55. [Crossref] [PubMed]
- Ornelas A, Zacharias-Millward N, Menter DG, et al. Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev 2017;36:289-303. [Crossref] [PubMed]
- Devi S, Wang Y, Chew WK, et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med 2013;210:2321-36. [Crossref] [PubMed]
- Puga I, Cols M, Barra CM, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 2011;13:170-80. [Crossref] [PubMed]
- Hampton HR, Chtanova T. The lymph node neutrophil. Semin Immunol 2016;28:129-36. [Crossref] [PubMed]
- Beauvillain C, Cunin P, Doni A, et al. CCR7 is involved in the migration of neutrophils to lymph nodes. Blood 2011;117:1196-204. [Crossref] [PubMed]
- Massena S, Christoffersson G, Vågesjö E, et al. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood 2015;126:2016-26. [Crossref] [PubMed]
- Silvestre-Roig C, Hidalgo A, Soehnlein O. Neutrophil heterogeneity: implications for homeostasis and pathogenesis. Blood 2016;127:2173-81. [Crossref] [PubMed]
- Yang F, Feng C, Zhang X, et al. The Diverse Biological Functions of Neutrophils, Beyond the Defense Against Infections. Inflammation 2017;40:311-23. [Crossref] [PubMed]
- Wang X, Qiu L, Li Z, et al. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front Immunol 2018;9:2456. [Crossref] [PubMed]
- Sagiv JY, Voels S, Granot Z. Isolation and Characterization of Low- vs. High-Density Neutrophils in Cancer. Methods Mol Biol 2016;1458:179-93. [Crossref] [PubMed]
- Sagiv JY, Michaeli J, Assi S, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 2015;10:562-73. [Crossref] [PubMed]
- Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [Crossref] [PubMed]
- Kargl J, Busch SE, Yang GHY, et al. Neutrophils dominate the immune cell composition in non-small cell lung cancer. Nat Commun 2017;8:14381. [Crossref] [PubMed]
- Condamine T, Dominguez GA, Youn JI, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol 2016;1:aaf8943 [Crossref] [PubMed]
- Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum 1986;29:1334-42. [Crossref] [PubMed]
- Krieg C, Shaul M, Guglietta S, et al. Circulating Low Density Neutrophils In Advanced Lung Cancer Patients Exhibit Unique Immune Signatures and affect prognosis. J Immunol 2020;204:164.11.
- Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176-81. [Crossref] [PubMed]
- Mishalian I, Bayuh R, Levy L, et al. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother 2013;62:1745-56. [Crossref] [PubMed]
- Ma Y, Yabluchanskiy A, Iyer RP, et al. Temporal neutrophil polarization following myocardial infarction. Cardiovasc Res 2016;110:51-61. [Crossref] [PubMed]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009;16:183-94. [Crossref] [PubMed]
- Jablonska J, Leschner S, Westphal K, et al. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest 2010;120:1151-64. [Crossref] [PubMed]
- Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000;2:737-44. [Crossref] [PubMed]
- Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol 2014;5:508. [Crossref] [PubMed]
- Kruger P, Saffarzadeh M, Weber AN, et al. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog 2015;11:e1004651 [Crossref] [PubMed]
- Vaguliene N, Zemaitis M, Lavinskiene S, et al. Local and systemic neutrophilic inflammation in patients with lung cancer and chronic obstructive pulmonary disease. BMC Immunol 2013;14:36. [Crossref] [PubMed]
- Vannitamby A, Hendry S, Irving L, et al. Novel multiplex droplet digital PCR assay for scoring PD-L1 in non-small cell lung cancer biopsy specimens. Lung Cancer 2019;134:233-7. [Crossref] [PubMed]
- Vannitamby A, Seow HJ, Anderson G, et al. Tumour-associated neutrophils and loss of epithelial PTEN can promote corticosteroid-insensitive MMP-9 expression in the chronically inflamed lung microenvironment. Thorax 2017;72:1140-3. [Crossref] [PubMed]
- Bissett D, O'Byrne KJ, von Pawel J, et al. Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small-cell lung cancer. J Clin Oncol 2005;23:842-9. [Crossref] [PubMed]
- Leighl NB, Paz-Ares L, Douillard JY, et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J Clin Oncol 2005;23:2831-9. [Crossref] [PubMed]
- Winer A, Adams S, Mignatti P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures Into Future Successes. Mol Cancer Ther 2018;17:1147-55. [Crossref] [PubMed]
- Fields GB. Mechanisms of Action of Novel Drugs Targeting Angiogenesis-Promoting Matrix Metalloproteinases. Front Immunol 2019;10:1278. [Crossref] [PubMed]
- Sarraf KM, Belcher E, Raevsky E, et al. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg 2009;137:425-8. [Crossref] [PubMed]
- Antosova M, Bencova A, Mikolka P, et al. The markers of oxidative stress in patient with lung cancer. Eur Respir J 2015;46:PA4267
- Aggarwal V, Tuli HS, Varol A, et al. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019;9:735. [Crossref] [PubMed]
- Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Molecular Cancer 2013;12:86. [Crossref] [PubMed]
- Gu L, Wang Z, Zuo J, et al. Prognostic significance of NF-κB expression in non-small cell lung cancer: A meta-analysis. PLoS One 2018;13:e0198223 [Crossref] [PubMed]
- Whiteside TL. Down-regulation of zeta-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother 2004;53:865-78. [Crossref] [PubMed]
- Kramer PA, Prichard L, Chacko B, et al. Inhibition of the lymphocyte metabolic switch by the oxidative burst of human neutrophils. Clin Sci (Lond) 2015;129:489-504. [Crossref] [PubMed]
- Hatanaka H, Abe Y, Kamiya T, et al. Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Ann Oncol 2000;11:815-9. [Crossref] [PubMed]
- De Vita F, Orditura M, Galizia G, et al. Serum interleukin-10 levels as a prognostic factor in advanced non-small cell lung cancer patients. Chest 2000;117:365-73. [Crossref] [PubMed]
- Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood 1993;81:2964-71. [Crossref] [PubMed]
- De Santo C, Arscott R, Booth S, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol 2010;11:1039-46. [Crossref] [PubMed]
- Mattarollo SR, Smyth MJ. A novel axis of innate immunity in cancer. Nat Immunol 2010;11:981-2. [Crossref] [PubMed]
- Anthony D, Seow HJ, Uddin M, et al. Serum amyloid A promotes lung neutrophilia by increasing IL-17A levels in the mucosa and γδ T cells. Am J Respir Crit Care Med 2013;188:179-86. [Crossref] [PubMed]
- Bozinovski S, Hutchinson A, Thompson M, et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:269-78. [Crossref] [PubMed]
- Sung HJ, Ahn JM, Yoon YH, et al. Identification and validation of SAA as a potential lung cancer biomarker and its involvement in metastatic pathogenesis of lung cancer. J Proteome Res 2011;10:1383-95. [Crossref] [PubMed]
- Chen JJ, Yao PL, Yuan A, et al. Up-regulation of tumor interleukin-8 expression by infiltrating macrophages: its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res 2003;9:729-37. [PubMed]
- Masuya D, Huang C, Liu D, et al. The intratumoral expression of vascular endothelial growth factor and interleukin-8 associated with angiogenesis in nonsmall cell lung carcinoma patients. Cancer 2001;92:2628-38. [Crossref] [PubMed]
- Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res 2009;69:1553-60. [Crossref] [PubMed]
- Miret JJ, Kirschmeier P, Koyama S, et al. Suppression of Myeloid Cell Arginase Activity leads to Therapeutic Response in a NSCLC Mouse Model by Activating Anti-Tumor Immunity. J Immunother Cancer 2019;7:32. [Crossref] [PubMed]
- Grzywa TM, Sosnowska A, Matryba P, et al. Myeloid Cell-Derived Arginase in Cancer Immune Response. Front Immunol 2020;11:938. [Crossref] [PubMed]
- Xu J, Acharya S, Sahin O, et al. 14-3-3ζ turns TGF-β's function from tumor suppressor to metastasis promoter in breast cancer by contextual changes of Smad partners from p53 to Gli2. Cancer Cell 2015;27:177-92. [Crossref] [PubMed]
- Akahira J, Sugihashi Y, Suzuki T, et al. Decreased expression of 14-3-3 sigma is associated with advanced disease in human epithelial ovarian cancer: its correlation with aberrant DNA methylation. Clin Cancer Res 2004;10:2687-93. [Crossref] [PubMed]
- Hasegawa Y, Takanashi S, Kanehira Y, et al. Transforming growth factor-beta1 level correlates with angiogenesis, tumor progression, and prognosis in patients with nonsmall cell lung carcinoma. Cancer 2001;91:964-71. [Crossref] [PubMed]
- Ye Y, Liu S, Wu C, et al. TGFβ modulates inflammatory cytokines and growth factors to create premetastatic microenvironment and stimulate lung metastasis. J Mol Histol 2015;46:365-75. [Crossref] [PubMed]
- Batlle E, Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019;50:924-40. [Crossref] [PubMed]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52-67. [Crossref] [PubMed]
- Liu Y, Zhang P, Li J, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol 2008;9:632-40. [Crossref] [PubMed]
- Akbay EA, Koyama S, Liu Y, et al. Interleukin-17A Promotes Lung Tumor Progression through Neutrophil Attraction to Tumor Sites and Mediating Resistance to PD-1 Blockade. J Thorac Oncol 2017;12:1268-79. [Crossref] [PubMed]
- Chung AS, Wu X, Zhuang G, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med 2013;19:1114-23. [Crossref] [PubMed]
- Reppert S, Boross I, Koslowski M, et al. A role for T-bet-mediated tumour immune surveillance in anti-IL-17A treatment of lung cancer. Nat Commun 2011;2:600. [Crossref] [PubMed]
- Wang R, Yang L, Zhang C, et al. Th17 cell-derived IL-17A promoted tumor progression via STAT3/NF-κB/Notch1 signaling in non-small cell lung cancer. Oncoimmunology 2018;7:e1461303 [Crossref] [PubMed]
- Chang SH, Mirabolfathinejad SG, Katta H, et al. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A 2014;111:5664-9. [Crossref] [PubMed]


