
Prognostic impact of PD-1 and PD-L1 expression in malignant pleural mesothelioma: an international multicenter study
Introduction
Malignant pleural mesothelioma (MPM) is a rare and aggressive malignancy arising from the pleural mesothelium. The overall survival (OS) of MPM patients ranges from 10 to 20 months depending on stage and histological subtype (1-4). Platinum-based chemotherapy (CHT) has been used in MPM treatment and still remains the backbone for current combination strategies (5). Recent advances in multidisciplinary therapeutic approaches including surgery, CHT and radiation therapy (RT) have improved the OS in highly selected patients (1,6-9). Moreover, recent phase I/II trials have shown some benefit of immunotherapy in MPM, but single agent checkpoint inhibitors were so far not demonstrated to be superior to standard CHT in larger phase III trials (10-12). Nevertheless, a recent phase III study investigating the efficacy of first-line nivolumab plus ipilimumab (vs. platinum doublet CHT) showed promising results with regards to OS (13). Of note, however, the progression-free survival (PFS) was similar between the treatment arms even in case of combination immunotherapy (13). Altogether, selecting MPM patients for appropriate therapeutic approaches remains a key problem, resulting in an unmet need to identify prognostic markers to predict the OS and to individualize treatment based on expected prognosis.
Besides their potential to predict the efficacy of immunotherapy, programmed death ligand 1 (PD-L1) and programmed cell death 1 (PD-1) expressions have shown conflicting results in correlating with prognosis in several tumor types (14). Specifically, high PD-L1 level was found to be a negative prognostic factor in renal and gastric cancers, while in primary colorectal and thymic carcinomas PD-L1 was associated with favourable outcome (14-16). In lung cancer, immunotherapy is a well-established first-line treatment and its use is based on the predictive role of PD-L1 expression (17). However, several studies have reported favourable whereas some have shown dismal outcome based on high PD1/PD-L1 expressions and thus the prognostic role of these tissue biomarkers to date remains unclear in lung cancer (18-21). In MPM, currently only limited data is available on the prevalence and prognostic role of PD-L1 and PD-1 expressions and the exact role of these tissue biomarkers in predicting MPM outcome remains thus controversial (15,22-25). To further explore the expression and prognostic impact of PD-L1 and PD-1 of tumor cells (TCs) and tumor-infiltrating lymphocytes (TILs), our multi-institutional study investigated the expression patterns of these molecules and their relationship with clinicopathologic parameters and long-term outcome in human MPM.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-1114).
Methods
Study population
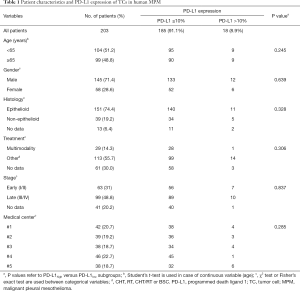
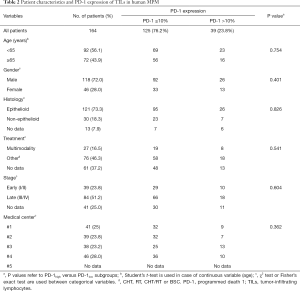
In this multicenter study, we included 203 patients with histologically confirmed MPM, diagnosed in the following 5 Central European medical centers between 2000 and 2016 (Tables 1,2): Department for Respiratory Diseases Jordanovac, University of Zagreb School of Medicine, Zagreb, Croatia (cohort #1, n=42); National Koranyi Institute of Pulmonology, Budapest, Hungary (cohort #2, n=39); Department of Thoracic Surgery, Medical University of Vienna, Austria (cohort #3, n=38); Department for Pulmonology, University Clinic Golnik, Golnik, Slovenia (cohort #4, n=46), and Department of Respiratory Medicine, University Hospital, Palacky University, Olomouc, Czech Republic (cohort #5, n=38). Only patients with adequate clinical data and sufficient amount of formalin-fixed paraffin-embedded (FFPE) tumor tissue were included. Staging was performed according to the IMIG TNM staging system (7th edition) (26). Clinical data regarding patient age, gender, clinical stage, histological subtype, treatment and survival data for the included patients were retrospectively collected from medical records and/or records from the National Health Insurance Offices or Central Statistical Offices of each participating country.
Full table
Full table
Tissue samples
Tissue samples were acquired during diagnostic procedures or surgery. The FFPE tissue specimens were routinely processed and examined for routine diagnostic work-up and consequently stored at room temperature. To re-confirm the diagnosis of MPM of each case and to evaluate tumor content, serial sections were cut at 4 μm and stained first with hematoxylin and eosin. Further sections were used for the immunohistochemical analyses. In 23 (11%) cases, samples were collected at the time of surgery after administration of neoadjuvant platinum-based CHT. All other samples were retrieved from CHT-naïve patients.
Immunohistochemistry
The monoclonal PD-L1 and PD-1 antibodies (Cell Signaling, clone E1L3N, dilution 1:25 and R&D Systems, # AF 1086, dilution 1:20, respectively) for immunostaining were used as reported by the standardized protocols of the ISO-certified laboratory of the 1st Department of Medicine, Medical University of Vienna. Briefly, after deparaffinization and rehydration of the 4 µm-thick sections, the slides were incubated in a 0.3% H2O2 solution for 10 minutes in order to reduce the nonspecific background staining, and heated afterwards for 10 minutes in 10 mM citrate buffer (pH 6.0) in a pressure cooker. Slides were then incubated for 5 minutes at room temperature with Ultra V Block (UltraVision LP detection system, Lab Vision Corporation, Thermo Fisher Scientific Inc., Pittsburgh, MA, USA), followed by PD-L1 and PD-1 antibody incubation for 30 minutes at room temperature. Antibody binding was detected by using The UltraVision LP detection system following the manufacturer’s recommendations (Lab Vision Corporation). Color development and antibody visualizations were performed with 3-3'-diaminobenzidine and counterstained with hematoxylin. PD-L1 and PD-1 expressions were examined blinded to clinical data of the patients by an expert pathologist. Slides were examined at 400× magnification, and the staining rate (percentage of all TCs and TILs showing positive staining) was determined. PD-L1 and PD-1 staining intensity was scored as 0 (absent), 1 (weak), 2 (moderate), and 3 (strong). The degrees of PD-L1 and PD-1 expressions were classified separately according to the percentage of positive TCs or TILs as follows: (TC low) 0–10% TCs positive, (TC high) >10% TCs positive, (TIL low) 0–10% TILs positive, (TIL high) >10% TILs positive.
Treatment
All diagnostic and therapeutic approaches were conducted in accordance with the individual institutional and with the current National Comprehensive Cancer Network (NCCN) guidelines with no major differences across the 5 institutions (27). Accordingly, after the diagnosis and tissue sampling, patients were treated with either platinum-based CHT, palliative radiotherapy (RT), combined chemoradiotherapy (CHT-RT) or best supportive care (BSC). Multimodality treatment (MMT) was defined as the combination of surgery by either pleurectomy/decortication (P/D) or extrapleural pneumonectomy (EPP) with either chemo- and/or radiotherapy. None of the included patients received immunotherapy or other targeted agents.
Statistical analyses
All statistical analyses were performed using the SPSS Statistics 26.0 package (SPSS Inc., Chicago, IL, USA) and GraphPad Prism Version 8. Data distribution was verified by the Kolmogorov-Smirnov normality test. Categorical and ordinal parameters such as gender (male vs. female), clinical stage (I−II vs. III−IV), histological subtype (epithelioid vs. non-epithelioid) and dichotomized PD-L1/PD-1 expression (low vs. high), were analysed by χ2 test or Fisher’s exact test. Age as a continuous variable was analysed in the different PD-L1/PD-1 subgroups by Mann-Whitney U test and Student’s t-test. The primary endpoint of our study was the OS, which was estimated from the time of diagnosis until death of any cause, or the last available follow-up visit. Follow-up was completed until March 2018. Survival curves were estimated by Kaplan-Meier plots and the differences between different groups were compared using the log-rank test. The independent prognostic value of the clinicopathological variables was studied with a multivariate Cox proportional hazard regression model, which was adjusted for PD-L1 expression, age (as a continuous variable), gender, IMIG clinical stage, histological type and therapeutic approaches. For an additional exploratory multivariate analysis, multiple imputations by chain equation (MICE) were employed to handle missing data, in order to avoid the omission of valuable information. Continuous data are always shown as median or mean and corresponding range or, in case of OS, as median and corresponding 95% confidence interval (CI). All reported P values are two-sided, and a level of 0.05 or less was considered statistically significant.
Ethics statement
The study was conducted in accordance with the guidelines of the Helsinki Declaration (as revised in 2013) of the World Medical Association and the Good Scientific Practice guidelines of the Medical University of Vienna with the approval of the national level ethics committee (Medical University of Vienna; EK#: 904/2009). Due to the retrospective nature of the study, the requirement for written informed consent was waived. Tissue and data collection were approved in all institutions. After clinical information was collected, patient identifiers were removed, and subsequently, patients could not be identified either directly or indirectly. Tissue staining and data analysis was performed at the Medical University of Vienna (center #3).
Results
Correlation of clinicopathological variables with PD-L1/PD-1 expression
In total, 203 MPM patients were enrolled in the study whose clinicopathological characteristics are summarized in Tables 1,2. The full cohort comprised 151 (74%) epithelioid and 39 (19%) non-epithelioid (i.e., biphasic or sarcomatoid) MPMs. Thirteen (6%) cases were classified as MPM not otherwise specified (NOS). Median age of all cases was 64 years (range, 27–86 years) and patients were predominantly male (71.4%). At diagnosis, 63 (31%) and 99 (49%) cases had IMIG/TNM stage I−II and stage III−IV disease, respectively. Twenty-nine (14%) patients received multimodality treatment including surgery (MMT), while 113 (56%) patients underwent other therapeutic approaches such as CHT, RT, CHT/RT or BSC. In case of 61 patients, treatment-related data was not available.
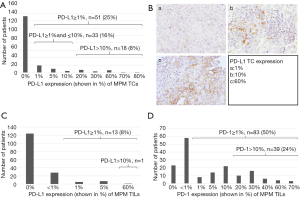
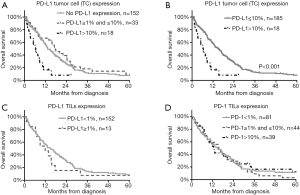
PD-L1 expression was measured in both of the TC and TIL populations. Meanwhile, PD-1 expression was analyzed solely in TILs because we did not observe any positivity on TCs. Out of all 203 cases, 152 (75%) cases did not show any TC PD-L1 expression. Of the 51 (25%) cases who were categorized as TC/PD-L1 positive (≥1%), the tumor samples of 33 (16%) and 18 (9%) patients were categorized by TC/PD-L1 scores “low” and “high”, respectively (Figure 1A). Representative images of PD-L1 expressions of TCs are shown in Figure 1B. Eligible MPM tissue for investigating PD-L1 expression of TILs was available from 165 patients. PD-L1 TIL expression was rarely seen. Positive staining (PD-L1 TIL expression ≥1%) was found in 13 (8%) patients, and only 1 case exhibited a PD-L1 TIL expression >10% (Figure 1C). PD-1 expression of TILs could be measured in 164 patients. TIL PD-1 positivity (i.e., ≥1%) was found in 83 (50%) patients. A higher than 10% TIL PD-1 expression was observed in 39 (24%) patients (Figure 1D).
Next, we studied the correlation between clinicopathological parameters and PD-L1 and PD-1 expression of TCs and TILs. No significant correlation was found between PD-L1 or PD-1 TC or TIL expressions and clinical variables such as age, gender, histological subtype or tumor stage when patients were dichotomized into PD-L1 and PD-1 negative (no staining) vs. positive (≥1% staining) categories. Of note, using cut-off values of 10% (Tables 1,2) or 50% (data not shown) for PD-L1 or PD-1 expressions did not yield significant associations either. It is also important to mention that we did not find significant associations between TC or TIL PD-L1/PD-1 expressions and histological subtypes or therapeutic modality (Tables 1,2).
Prognostic parameters and OS
The median follow-up time for all 203 patients was 12.8 months. Median OS of the full cohort was 13.2 months (95% CI, 10.6–15.8). First, we performed a univariate survival analysis in order to identify clinical prognostic factors for OS (Table 3). We found that patients with epithelioid histological subtype exhibited significantly improved OS compared to those with non-epithelioid MPM (median OSs were 13.2 vs. 12.7 months, respectively; HR 0.64, P=0.012, Figure 2A). Patients with stage I/II MPM (vs. stage III/IV, respectively, HR 0.66, P=0.01, Table 3 and Figure 2B) and patients receiving MMT (vs. other therapies, HR 0.32, P<0.001, Table 3 and Figure 2C) were also associated with significantly improved OS. There were no significant associations between OS and gender (Figure 2D) or age (dichotomized at a cut-off of 65 years, data not shown).

Full table
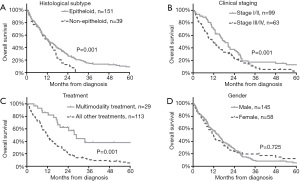
Next, we examined the prognostic value of PD-L1 and PD-1 expression of TCs and TILs (Table 3). Our initial statistical analyses indicated that patients whose TCs did not express PD-L1 (median OS 14 months) had comparable OS to those with PD-L1 TC expressions between 1% and 10% (median OS 16 months, P=0.194, Figure 3A). We grouped patients accordingly into low (≤10%) and high (>10%) PD-L1 TC categories and found that low PD-L1 expression was significantly associated with improved OS (HR 0.39, P<0.001, Table 3 and Figure 3B). PD-L1 was rarely expressed by TILS and there was no difference in the OS of patients whose tumor samples were categorized by a PD-L1TIL score <1% (n=152) vs. ≥1% (median OSs were 15.1 vs. 11.8 months, HR 0.82, P=0.508, Table 3 and Figure 3C). Similarly, we could not show prognostic information from the PD-1 expression of TILs when patients were grouped into PD-1 TIL <1% vs. ≥1% and ≤10% vs. >10% categories (Table 3 and Figure 3D).
In order to assess if the prognostic value of PD-L1 TC expression was independent from significant clinical prognostic factors, we performed a multivariate Cox regression analysis with available data from 126 (62%) patients (Table 4). The model was adjusted for clinical factors such as age, gender, histological subtype, tumor stage at diagnosis and treatment. We found that PD-L1 TC expression at a 10% cut-off remained a significant prognostic factor for OS (low vs. high expression; HR 0.405, P=0.005; Table 4). Histological subtype (epithelioid vs. non-epithelioid; HR 0.504, P=0.009), tumor stage (I−II vs. III-IV; HR 0.545, P=0.007) and treatment (MMT vs. other therapies, HR 0.351, P<0.001) also independently influenced OS. As 126 (62%) patients only had completely available data for the multivariate model, we performed an exploratory multivariate Cox regression analysis, using a dataset after multiple imputation by MICE approach, including all 203 cases, in order to avoid the omission of data. In this exploratory analysis, PD-L1 TC expression remained as a significant prognostic factor for OS (HR 0.443, P=0.004), independent from age, gender, histologic subtype, stage and treatment (data not shown).

Full table
Discussion
The poor survival outcome in MPM and the lack of effective therapies require the development of novel therapeutic strategies. Hence, there is an urgent need for identifying specific prognostic and predictive markers that enable clinicians to effectively allocate patients to appropriate treatment. Previous studies suggest that high PD-L1 expression might be associated with impaired survival outcomes in MPM, yet the prognostic value and clinicopathological significance of both PD-L1 and PD-1 are still controversial (1,15,24). Therefore, the aim of this study was to evaluate the expression of PD-L1 and its receptor PD-1 in MPM and to correlate their expression patterns with clinicopathological parameters and long-term outcome by analyzing a large patient cohort in a multicenter setting.
The majority of MPM cases are caused by prior exposure to asbestos leading to the increase of local infiltrating immune cells and malignant transformation of mesothelial cells (22,28,29). High numbers of TILs have been associated with a better prognosis, while high numbers of TAMs and low lymphocyte to monocyte ratio (LMR) in peripheral blood or tissue have a negative impact on prognosis (2,22,30-32). The PD-L1/PD-1 pathway plays a pivotal role in normal immune system regulation, but also in tumor immune escape control since the interaction of TC PD-L1 with T-cell PD-1 reduces the effector functions of T cells (33). Accordingly, immunogenic tumors can easily bypass the anti-tumor responses of the organism by overexpressing PD-L1, and thus escaping the immune surveillance (33). On the other hand, by blocking the PD-L1/PD-1 pathway with therapeutic antibodies a durable anti-tumor activity and favorable response rates can be achieved in multiple tumor types, including skin melanoma, lung cancer, and partly in MPM as well (14,34).
In our international multicenter study, we found that 25% of cases were categorized as positive (≥1%) for TC PD-L1 expression. These results are in agreement with two recent MPM studies reporting that 18% to 24% of the patients had PD-L1 expressing tumors (24,35). Additionally, we found that only a small number of patients (n=18; 8%) had a PD-L1 TC expression higher than 10%. Previous studies have shown that PD-L1 can be expressed by multiple components of the tumor microenvironment. Therefore, we investigated PD-L1 expression by TILs too (24). However, PD-L1 was rarely expressed by TILs in our cohort. These results are only partly in line with the findings of Herbst and colleagues, who studied 732 different tumor types and observed PD-L1 positivity on both TCs and immune cells (36). A possible explanation for the relatively low number of cases with PD-L1 expressing TILs might be that TIL PD-L1 positivity is usually seen in sarcomatoid MPM, whereas the majority of patients included in our study had epithelioid type MPM (37).
So far, two major studies investigated the detailed expression pattern of PD-1 in MPM (37,38). In our study, PD-1 expression of TILs could be measured in 164 patients, whereas we did not observe any PD-1 positive TCs. Our results are in line with the findings of Marcq and colleagues who demonstrated that PD-1 is expressed to a great extent on immune cells in MPM (37). Additionally, they also showed that PD-1 positive TCs are rarely seen in these patients (only 4 of 54 patients had PD-1 positive TCs in their study) (37). PD-1 is primarily expressed by activated lymphocytes and upon triggering by its ligands (PD-L1 and PD-L2) it can repress Th1 cytotoxic immune responses (14,39). Interestingly, half of our patients have been categorized as positive for TIL PD-1 expression, and 24% of them had high (>10%) PD-1 expression. Interestingly, the significance of PD-1-expressing tumor infiltrating CD8+ T cells in predicting anti-PD-1 therapeutic response in MPM is still unclear (40). Of note, in case of other solid tumors, such as skin melanoma it is suspected that the presence of activated PD-1+ CD8+ T cells might be associated with therapeutic efficacy (40,41). As mentioned before, previous studies have reported higher PD-L1 expression in non-epithelioid (especially sarcomatoid) MPM compared to other histological subtypes (37,38). We did not find a significant association between PD-L1 or PD-1 expression and histological subtype. Our results are, therefore, in contrast to previous studies (23,24,38). A possible explanation for this discordance might be related to different cut-off values. In our study, “PD-L1/PD-1 high” patients were defined as those with PD-L1/PD-1 expression >10%. Meanwhile, others used alternative threshold values or grouped the patients solely based on positivity irrespective of the expression percentage. Additionally, the relatively low ratio of patients with non-epithelioid MPM in our study might also explain these divergent results.
To date, no threshold PD-L1 expression level predicting treatment response or survival probability in MPM has been defined (15). In contrast to previous studies applying cut-off levels of 1% or 5% (15,24,42,43), in the present study we investigated the correlation between PD-L1 expression and OS using cut-off levels of 1% and 10%. PD-L1 and PD-1 expression have been shown to correlate with survival in several tumor types including hepatocellular, breast, esophageal and thymic carcinomas (14,16,44-46). As for MPM, the small number of available studies has yielded conflicting results partly due to different threshold values (15,24,35,42,43). In our study, we found that high (>10%) TC PD-L1 expression was associated with impaired median OS, with a clinically relevant difference of 8.8 months between low and high subgroups. In addition, by performing multivariate analysis, high (>10%) PD-L1 expression was significantly associated with OS, regardless of histology, stage or treatment. Of note, similar survival probabilities between PD-L1 negative patients (<1%) and those with PD-L1 expression between 1–10% might suggest the need for higher cut-off values compared to previous studies. PD-L1 protein expression was previously shown to correlate with tumor aggressiveness and may be a critical factor to promote tumor growth and metastases (1,24,46-48). Accordingly, the worse OS related to higher PD-L1 TC expression levels may be partly explained by PD-L1 acting as a surrogate marker for an unfavorable tumor behavior.
As for the prognostic impact of PD-1 expression by TILs, previous studies suggest that PD-1 expression by immune cells correlates with increased OS in patients with triple-negative breast cancer, gastric cancer or skin melanoma (40,49,50). Meanwhile, in case of other solid tumors including nasopharyngeal carcinoma no such association was found (51). To the best of our knowledge, ours is so far the largest study to investigate the prognostic relevance of PD-1 expression by TILs in MPM patients. Although Marcq et al. also examined the prognostic importance of PD-1 on immune cells, their study included only 54 patients (37). In the present study, we could not detect any statistically or clinically relevant difference in the OS of subcohorts with different PD-1 TILs expressions. Accordingly, our results suggest that PD-1 TIL expression may not serve as a suitable prognostic biomarker in MPM.
The present study is partly limited by its retrospective nature and also by the lack of a validation set. Accordingly, results have to be interpreted with caution. Additionally, the use of PD-L1 expression as a prognostic biomarker can be confounded by multiple unresolved issues, including variability in antibody characteristics, tissue processing and expression threshold values. In this study, we used the commercially available E1L3N antibody for PD-L1 staining. Importantly, however, not all antibody clones show a similar staining pattern and positivity (52). Therefore, our results should preferentially be considered when using the E1L3N antibody clone. Finally, our results should be interpreted with the caveat that both PD-L1 and PD-1 expressions are variable over time, and although the majority of included patients were CHT-naïve at biopsy, the application of CHT prior to tissue sampling can possibly confound expression patterns (37,53,54). Nevertheless, this study examined a relatively large number of patients in a multicenter setting and we used multiple cut-off values in order to get a clearer insight into the expression pattern and prognostic impact of both PD-L1 and PD-1.
To conclude, the results of this study show that PD-L1 is uniformly expressed by both TCs and TILs in MPM. Furthermore, this is the largest study that comprehensively evaluates the prognostic value of PD-1 by TILs in a multicenter cohort of MPM patients. Our study also demonstrates that high (>10%) TC PD-L1 expression is associated with a clinically relevant survival disadvantage and, furthermore, that it is an independent prognostic factor in MPM. Altogether, by shedding light on the expression patterns and prognostic relevance of PD-L1 and PD-1 our results might provide support for further MPM trials investigating PD-L1/PD-1 inhibition, especially in patients with high PD-L1 expression and consequently poor survival.
Acknowledgments
Funding: TK was supported by the Medical Scientific Fund of the Mayor of the City of Vienna (#17028). SS was partly supported by Croatian Science Foundation (grant IP-2014-09-4173). BD, BH and VL were supported by the Austrian Science Fund (FWF I2872, BH; FWF I3522, VL; FWF I3977 and I4677, BD). JF acknowledges funding from the Hungarian National Research, Development and Innovation Office (ANN128666). VL is a recipient of the Bolyai Research Scholarship of the Hungarian Academy of Sciences and the UNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology. ZM was supported by the UNKP-20-3 New National Excellence Program of the Ministry for Innovation and Technology.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-1114
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-1114
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-1114
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-1114). TK was supported by the Medical Scientific Fund of the Mayor of the City of Vienna (#17028). SS was partly supported by Croatian Science Foundation (grant IP-2014-09-4173). BD, BH and VL were supported by the Austrian Science Fund (FWF I2872, BH; FWF I3522, VL; FWF I3977 and I4677, BD). JF acknowledges funding from the Hungarian National Research, Development and Innovation Office (ANN128666). VL is a recipient of the Bolyai Research Scholarship of the Hungarian Academy of Sciences and the UNKP-19-4 New National Excellence Program of the Ministry for Innovation and Technology. ZM was supported by the UNKP-20-3 New National Excellence Program of the Ministry for Innovation and Technology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the guidelines of the Helsinki Declaration (as revised in 2013) of the World Medical Association and the Good Scientific Practice guidelines of the Medical University of Vienna with the approval of the national level ethics committee (Medical University of Vienna; EK#: 904/2009). Due to the retrospective nature of the study, the requirement for written informed consent was waived. Tissue and data collection were approved in all institutions. After clinical information was collected, patient identifiers were removed, and subsequently, patients could not be identified either directly or indirectly. Tissue staining and data analysis was performed at the Medical University of Vienna (center #3).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cedres S, Ponce-Aix S, Pardo-Aranda N, et al. Analysis of expression of PTEN/PI3K pathway and programmed cell death ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). Lung Cancer 2016;96:1-6. [Crossref] [PubMed]
- Khanna S, Thomas A, Abate-Daga D, et al. Malignant Mesothelioma Effusions Are Infiltrated by CD3(+) T Cells Highly Expressing PD-L1 and the PD-L1(+) Tumor Cells within These Effusions Are Susceptible to ADCC by the Anti-PD-L1 Antibody Avelumab. J Thorac Oncol 2016;11:1993-2005. [Crossref] [PubMed]
- Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med 2005;353:1591-603. [Crossref] [PubMed]
- Rusch VW, Chansky K, Kindler HL, et al. The IASLC Mesothelioma Staging Project: Proposals for the M Descriptors and for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Mesothelioma. J Thorac Oncol 2016;11:2112-9. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016;14:825-36. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the "SMART" approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Lauk O, Hoda MA, de Perrot M, et al. Extrapleural Pneumonectomy After Induction Chemotherapy: Perioperative Outcome in 251 Mesothelioma Patients From Three High-Volume Institutions. Ann Thorac Surg 2014;98:1748-54. [Crossref] [PubMed]
- de Gooijer CJ, Borm FJ, Scherpereel A, et al. Immunotherapy in Malignant Pleural Mesothelioma. Front Oncol 2020;10:187. [Crossref] [PubMed]
- Okada M, Kijima T, Aoe K, et al. Clinical Efficacy and Safety of Nivolumab: Results of a. Clin Cancer Res 2019;25:5485-92. [Crossref] [PubMed]
- Gray SG, Mutti L. Immunotherapy for mesothelioma: a critical review of current clinical trials and future perspectives. Transl Lung Cancer Res 2020;9:S100-19. [Crossref] [PubMed]
- Baas P, Scherpereel A, Nowak A, et al. ID:2908 First-Line Nivolumab + Ipilimumab vs Chemotherapy in Unresectable Malignant Pleural Mesothelioma: CheckMate 743. J Thorac Oncol 2020;15:e42 [Crossref]
- Kollmann D, Schweiger T, Schwarz S, et al. PD1-positive tumor-infiltrating lymphocytes are associated with poor clinical outcome after pulmonary metastasectomy for colorectal cancer. Oncoimmunology 2017;6:e1331194 [Crossref] [PubMed]
- Nguyen BH, Montgomery R, Fadia M, et al. PD-L1 expression associated with worse survival outcome in malignant pleural mesothelioma. Asia Pac J Clin Oncol 2018;14:69-73. [Crossref] [PubMed]
- Yokoyama S, Miyoshi H, Nakashima K, et al. Prognostic Value of Programmed Death Ligand 1 and Programmed Death 1 Expression in Thymic Carcinoma. Clin Cancer Res 2016;22:4727-34. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Aggarwal C, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 1.2020. J Natl Compr Canc Netw 2019;17:1464-72. [Crossref] [PubMed]
- Hulsbergen AFC, Mammi M, Nagtegaal SHJ, et al. Programmed Death Receptor Ligand One Expression May Independently Predict Survival in Patients With Non-Small Cell Lung Carcinoma Brain Metastases Receiving Immunotherapy. Int J Radiat Oncol Biol Phys 2020;108:258-67. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer 2016;57:91-103. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [Crossref] [PubMed]
- Takada K, Okamoto T, Toyokawa G, et al. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer 2017;104:7-15. [Crossref] [PubMed]
- Pasello G, Zago G, Lunardi F, et al. Malignant pleural mesothelioma immune microenvironment and checkpoint expression: correlation with clinical-pathological features and intratumor heterogeneity over time. Ann Oncol 2018;29:1258-65. [Crossref] [PubMed]
- Brosseau S, Danel C, Scherpereel A, et al. Shorter Survival in Malignant Pleural Mesothelioma Patients With High PD-L1 Expression Associated With Sarcomatoid or Biphasic Histology Subtype: A Series of 214 Cases From the Bio-MAPS Cohort. Clin Lung Cancer 2019;20:e564-75. [Crossref] [PubMed]
- Cedres S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One 2015;10:e0121071 [Crossref] [PubMed]
- Remon J, Passiglia F, Ahn MJ, et al. Immune checkpoint inhibitors in thoracic malignancies: Review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol 2020;15:914-47. [Crossref] [PubMed]
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995;108:1122-8. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016;14:825-36. [Crossref] [PubMed]
- Bibby AC, Tsim S, Kanellakis N, et al. Malignant pleural mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir Rev 2016;25:472-86. [Crossref] [PubMed]
- Carbone M, Yang H. Molecular pathways: targeting mechanisms of asbestos and erionite carcinogenesis in mesothelioma. Clin Cancer Res 2012;18:598-604. [Crossref] [PubMed]
- Suzuki K, Kadota K, Sima CS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother 2011;60:1721-8. [Crossref] [PubMed]
- Ujiie H, Kadota K, Nitadori JI, et al. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015;4:e1009285 [Crossref] [PubMed]
- Yamagishi T, Fujimoto N, Nishi H, et al. Prognostic significance of the lymphocyte-to-monocyte ratio in patients with malignant pleural mesothelioma. Lung Cancer 2015;90:111-7. [Crossref] [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 2014;9:1036-40. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Marcq E, Siozopoulou V, De Waele J, et al. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 2016;6:e1261241 [Crossref] [PubMed]
- Combaz-Lair C, Galateau-Salle F, McLeer-Florin A, et al. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum Pathol 2016;52:9-18. [Crossref] [PubMed]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol 2004;4:336-47. [Crossref] [PubMed]
- Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology 2017;7:e1364828 [Crossref] [PubMed]
- Johnson DB, Estrada MV, Salgado R, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun 2016;7:10582. [Crossref] [PubMed]
- Thapa B, Salcedo A, Lin X, et al. The Immune Microenvironment, Genome-wide Copy Number Aberrations, and Survival in Mesothelioma. J Thorac Oncol 2017;12:850-9. [Crossref] [PubMed]
- Kao SC, Cheng YY, Williams M, et al. Tumor Suppressor microRNAs Contribute to the Regulation of PD-L1 Expression in Malignant Pleural Mesothelioma. J Thorac Oncol 2017;12:1421-33. [Crossref] [PubMed]
- Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9. [Crossref] [PubMed]
- Muenst S, Soysal SD, Gao F, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2013;139:667-76. [Crossref] [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Konishi J, Yamazaki K, Azuma M, et al. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004;10:5094-100. [Crossref] [PubMed]
- Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009;15:6341-7. [Crossref] [PubMed]
- Saito H, Shimizu S, Kono Y, et al. PD-1 Expression on Circulating CD8(+) T-Cells as a Prognostic Marker for Patients With Gastric Cancer. Anticancer Res 2019;39:443-8. [Crossref] [PubMed]
- Yeong J, Lim JCT, Lee B, et al. Prognostic value of CD8 + PD-1+ immune infiltrates and PDCD1 gene expression in triple negative breast cancer. J Immunother Cancer 2019;7:34. [Crossref] [PubMed]
- Huang ZL, Liu S, Wang GN, et al. The prognostic significance of PD-L1 and PD-1 expression in patients with nasopharyngeal carcinoma: a systematic review and meta-analysis. Cancer Cell Int 2019;19:141. [Crossref] [PubMed]
- Ma J, Li J, Qian M, et al. PD-L1 expression and the prognostic significance in gastric cancer: a retrospective comparison of three PD-L1 antibody clones (SP142, 28–8 and E1L3N). Diagnostic Pathology 2018;13:91. [Crossref] [PubMed]
- Guo L, Song P, Xue X, et al. Variation of Programmed Death Ligand 1 Expression After Platinum-based Neoadjuvant Chemotherapy in Lung Cancer. J Immunother 2019;42:215-20. [Crossref] [PubMed]
- Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep 2016;6:20090. [Crossref] [PubMed]


