
The effectiveness of three-dimensional reconstruction in the localization of multiple nodules in lung specimens: a prospective cohort study
Introduction
Since the mid-20th century, evidence supporting the substantial contribution of lung cancer screening to the detection and treatment of precursor lesions has been accumulating (1,2). With the improvement in awareness of cancer prevention and the clinical application of high-resolution computed tomography (CT), the number of patients diagnosed with multiple pulmonary nodules has gradually increased (3-5). In most cases, the lesions of these patients show as pure ground-glass nodules (pGGNs) or mixed ground-glass nodules (mGGNs) on CT images. For most patients with multiple nodules, surgery constitutes an effective radical treatment, and wedge resection, anatomical segmentectomy or lobectomy should be selected according to the particular case. Besides, surgical specimens can provide more detailed pathological and genetic characterization for follow-up treatment and monitoring of multiple primary lung cancers (6,7).
The reliability of sampling tissue specimens with multiple nodules depends on the precise location of each nodule. The ability of the pathologist to accurately determine the size and type of different nodules under the microscope can assist in clarifying the nature of multiple lesions, which is of guiding significance for tumor staging (multiple primary or intrapulmonary metastasis). Moreover, the level of agreement between the pathological report and preoperative CT imaging results can be helpful in assessing the accuracy of preoperative imaging diagnosis and informing further research on multiple pulmonary nodules.
However, in practice, it is challenging for pathologists to locate all nodules in specimens owing to a lack of understanding of preoperative CT images. Moreover, due to a lack of accurate description of the location of individual nodules, pathological reports for multiple nodules often fail to correspond with the findings of preoperative imaging. Therefore, it is urgent to find a method that will not only assist surgeons and pathologists to localize all nodules in resected specimens, but will also help researchers to obtain good agreement between pathological and imaging features in future studies (7).
Three-dimensional (3D) reconstruction and 3D printing models can provide cross-reference to guide surgeons and pathologists in locating multiple lesions in tissue specimens. We conducted a prospective cohort study to explore the performance of using 3D reconstruction/printing to locate synchronous multiple pulmonary nodules in resected specimens. This method may provide a novel perspective on the application of 3D reconstruction/3D printing models in pathology.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-21-202).
Methods
Study design
This study was conducted between September 2019 and September 2020 at the National Cancer Center (Beijing, China). The present study was designed to assess the value of using 3D reconstruction/printing in locating synchronous multiple pulmonary nodules in resected specimens. The study protocol is set out in Appendix 1. The study complies with the Declaration of Helsinki (as revised in 2013) and was approved by the Ethics Committee of the Cancer Hospital at the Chinese Academy of Medical Sciences (approval no. 20/130-2326).
Inclusion and exclusion criteria
Patients with synchronous multiple pulmonary nodules who were scheduled for anatomical segmentectomy or lobectomy were assessed by a trained researcher (Y Ji) to evaluate their eligibility for inclusion. The 2 main inclusion criteria were: largest diameter of the main lesion on the lung window ≤20 mm; and 2 or more lesions present in the same lobe or segment. The 2 primary exclusion criteria were: multiple lesions suspected to be metastatic tumors by preoperative evaluation; and conversion to wedge resection during the operation. The detailed inclusion and exclusion criteria are described in Appendix 1. Informed consent was taken from all the patients.
Primary outcome and secondary outcome
The primary outcome was the success rate of nodule localization in the resected specimens, and the secondary outcome was the agreement rate between the pathological results of the samples and CT images.
The total number of nodules was defined as the number of nodules planned to be resected based on preoperative CT scan. The nodules were located with the auxiliary of personalized 3D reconstruction imaging after surgical resection of the specimens. After all nodules were identified on the surgical specimens, and the corresponding areas were marked with 4.0 silk suture. The procedural duration was calculated as the time between specimens were resected and all nodules were labeled. Then, the labeled surgical specimens and the 3D printed models were sent to the Department of Pathology for sampling. The success rate of nodule localization was calculated as the ratio of the number of nodules actually localized to the total number of nodules based on preoperative imaging.
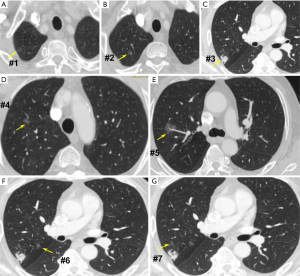
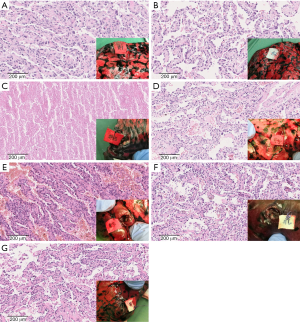
For example, Figure 1 shows the CT images of a 66-year-old man with 7 nodules in the right upper lobe. The 3D reconstruction/printing image and surgical specimen of this case are shown in Figure 2. The pathologist diagnosed each nodule according to the serial number (Figure 3).
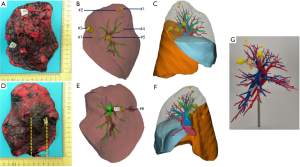
Preoperative 3D image construction and 3D model printing
The preoperative CT data of each patient enrolled in our study were obtained from the imaging workstation in our institution. The assessment of lesion composition was performed by an experienced radiologist (L Qi). The digital imaging and communications in medicine (DICOM) data of thin-slice (0.625–1.25 mm) CT images were imported into Mimics Software for 3D reconstruction. In all 40 cases, the 3D models were printed after processing of the reconstruction images in the ‘stereolithographic (stl)’ format with 3-matic Software. All models were fabricated by ProJet MJP 3600 (3d systems, USA) with VisiJet Proplast. Stereo lithography Appearance (SLA) technology was used to complete entity printing.
Pathologic diagnosis
The pathologic diagnosis of lung cancer was based on the 2015 World Health Organization (WHO) classification for lung cancer, and the staging standard was based on the 8th editions of the International Union Against Cancer and American Joint Committee on Cancer (UICC/AJCC) TNM staging system for non-small cell lung cancer (8,9). All histologic preparations and analyses were performed by 2 senior pathologists majoring in lung cancer (L Yang and X Wang). In case of disagreement, mutual consensus was reached after discussion with other pathologists.
Statistical analysis
Normally distributed continuous variables are presented as mean ± standard deviation (SD), and continuous variables that were not normally distributed are presented as median and interquartile range (25th, 75th percentile). Normality was assessed by the Shapiro-Wilk test and normal probability plot. Categorical variables are presented as frequency (percentage).
Results
In total, 43 patients with multiple pulmonary nodules treated in our hospital between September 1, 2019 and September 30, 2020 were eligible for inclusion in this study. Of them, 40 patients underwent lobectomy or anatomical segmentectomy were finally enrolled for analysis. A flowchart of participant enrollment is shown in Figure 4.
Patients characteristics
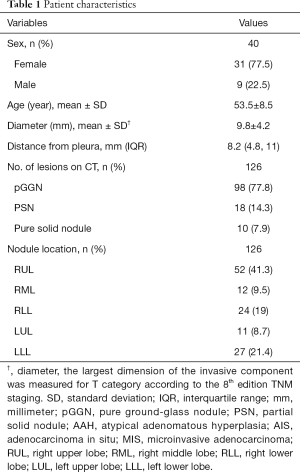
The 40 study participants included 9 male and 31 females, who had an average age of 53.5 (±8.5) years old. The 40 patients had a total of 126 nodules detected on CT, and the average lesion diameter was 9.8±4.2 mm. There were 98 (77.8%) pGGNs, 18 (14.3%) partial solid nodules (PSNs), and 10 (7.9%) pure solid nodules. The percentage of pGGNs is 70.4 (19/27) in male, while 79.8 (79/99) in female. For each patient, 1 or more lesions were not visible or could not be palpated on the surface of the surgically resected specimens. The median distance from the surface of the visceral pleura to the nodule was 8.2 mm (interquartile range, 25–75%, 4.8–11 mm) (Table 1). More details of each patient are shown in Table S1.
Full table
Characteristics of nodule localization
Of all 126 nodules of the 40 participants, 124 were successfully localized using the 3D reconstruction/printing models without sustaining damage. Thus, the success rate of nodule localization in the resected specimens was 98.4% (124/126). For the 124 successfully located nodules, the agreement rate of samples with CT images was 100% with the assistance of the 3D reconstruction/printing models. The mean procedural duration was 11 (±4.6) minutes (Figure 5).
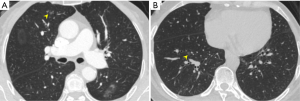
Surgical and pathological results are summarized in Table 2. Two pGGNs were not found (one in the right upper lobe, measuring 4.2 mm in the largest dimension; and the other, in the right lower lobe, measuring 5.8 mm in the largest dimension) (Figure 6).
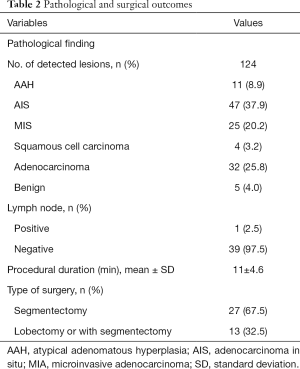
Full table
Discussion
Multiple primary lung cancer is a common and complex type of lung cancer. Due to the substantial variability of tumor characteristics and the combination of different sites of lung cancer, the characteristics of these tumors are highly complex, which greatly increases the difficulty of prognostic research of multiple primary lung cancer (8). In the 8th edition of the TNM staging classification for lung cancer, the Staging and Prognostic Factors Committee (SPFC) of the AJCC divides multiple pulmonary sites of lung cancer into the following 4 patterns: second primary lung cancers; multiple lung cancer nodules with prominent ground glass or lepidic (GG/L) features; lung cancer that is radiologically similar to pneumonia (i.e., pneumonic type); and intrapulmonary metastasis (9). With the popularization of CT screening, patients with multiple nodules of less than 1 cm are being diagnosed with increasing frequency. Sometimes, the lesions of a patient may contain both intrapulmonary metastasis and heterogeneous multiple primary lung cancer nodules. Thus, reliable methods to locate all nodules in resected specimens are urgently needed by surgeons and pathologists.
To date, various localization methods have been reported, including preoperative and intraoperative radio-guided detection, intraoperative ultrasound, and electromagnetic navigation (10-12). Preoperative CT-guided percutaneous fine-needle localization is an invasive operation, which may cause hemorrhage, pneumothorax, tumor translocation, and other complications (13). Moreover, not all cases are suitable for this method due to the position of lesions. Intraoperative ultrasound and electromagnetic navigation require the operator to be experienced and proficient in endoscopic devices. Besides, some special medical equipment is needed. Li et al. reported that CT-guided location of lesions in surgical specimens using fine needles under constant, moderate mechanical aeration allows for the rapid and accurate localization of lesions (14). However, this method has considerable limitations and its popularization in clinical practice is challenging. The success of their approach is highly dependent on the airway integrity of the surgical specimens. Thus, it requires inflatable aerator and CT scanner. The fact that the precise locations of multiple nodules are not usually indicated clearly in the pathological report greatly hinders retrospective studies of multiple primary lung cancer that are yet to be carried out, and this is deserving of more attention. Therefore, reference to the imaging information of each nodule is of great significance to improving the accuracy of pathological sampling and diagnosis.
If the nodules are not peripheral pulmonary lesions, then determining the pathological location of most GGNs in resected specimens is difficult. Moreover, it is harder to accurately identify the positions of these GGNs when they are located in the same lobe or segment, especially if they are adjacent to each other. At worst, the thoracic surgeon may be unable to find the nodule in the resected specimen, and serial sectioning of the whole specimen may be required to check for possible lesions. This scenario is a drain on time, and might even affect the scope of a given operation, pathological diagnosis, or the subsequent treatment strategy of the patient (15). With the auxiliary of 3D reconstruction/printing, we successfully located lesions in the resected specimens of all 40 cases. The important guiding significance of this method in the pathological location of multiple pulmonary nodules in resected specimens can be summarized as follows: (I) 3D reconstruction/printing models can improve the positive rate of pathological sampling of multiple pulmonary nodules; (II) through 3D reconstruction, the description of the position of nodules is more accurate and comprehensive than that in pathological reports; (III) 3D reconstruction/printing models can be used as a well-tried intermediate tool to match CT imaging features with the pathological features of multiple pulmonary nodules; (IV) 3D reconstruction/printing models can aid pathologists in gaining an understanding the characteristics of lesions in their entirety. The possible reasons why 2 of the pGGNs in this study could not be located is that the nodules were too small to be found in their respective specimens. However, given they were secondary lesions and lobectomy was performed for main foci, the failure to locate them did not affect the patients’ subsequent diagnosis and treatment.
It cannot be denied that there are still some limitations to this study. First of all, 3D reconstruction/printing is not suitable for wedge resection specimens, as they lack anatomical landmarks. Secondly, considering the expense of 3D printing model, further study should be implemented to evaluate the cost-effectiveness of 3D printing model.
Conclusions
In conclusion, we showed that locating multiple nodules in resected specimens with the auxiliary of 3D reconstruction has the success rate of 98%. This method represents novel progress in pathological sampling of multifocal pulmonary nodules and may have widespread availability. Furthermore, the excellent agreement between the pathological results of the samples and their CT images will provide assistance for future studies on the pathological characteristics and radiomics of multiple primary lung tumors.
Acknowledgments
The authors appreciate the academic support from AME Lung Cancer Collaborative Group. The authors also give thanks to Songtao Liu, ME, from Zhen Yuan (Tianjin) Medical Device Technology Co., Ltd. for providing technical support for the 3D printing technology, and to Jing Zhou, PhD, School of Statistics, Renmin University of China, for providing advice on the statistical design and data analysis.
Funding: This work was supported by the National Key R&D Program of China (2017YFC1308700), Institutional Fundamental Research Funds (2018PT32033), and the Graduate Innovation Fund of Peking Union Medical College (2019-1002-53).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-21-202
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-21-202
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-21-202). The authors report that receive only technological supports from Zhen Yuan (Tianjin) Medical Device Technology Co., Ltd., with no known financial stake. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (approval no. 20/130-2326). Informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2019;69:184-210. [Crossref] [PubMed]
- Wender RC, Brawley OW, Fedewa SA, et al. A blueprint for cancer screening and early detection: Advancing screening's contribution to cancer control. CA Cancer J Clin 2019;69:50-79. [Crossref] [PubMed]
- Asamura H. Multiple primary cancers or multiple metastases, that is the question. J Thorac Oncol 2010;5:930-1. [Crossref] [PubMed]
- Tanvetyanon T, Boyle TA. Clinical implications of genetic heterogeneity in multifocal pulmonary adenocarcinomas. J Thorac Dis 2016;8:E1734-8. [Crossref] [PubMed]
- Vazquez M, Carter D, Brambilla E, et al. Solitary and multiple resected adenocarcinomas after CT screening for lung cancer: histopathologic features and their prognostic implications. Lung Cancer 2009;64:148-54. [Crossref] [PubMed]
- Zhang Z, Gao S, Mao Y, et al. Surgical Outcomes of Synchronous Multiple Primary Non-Small Cell Lung Cancers. Sci Rep 2016;6:23252. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Shimizu S, Yatabe Y, Koshikawa T, et al. High frequency of clonally related tumors in cases of multiple synchronous lung cancers as revealed by molecular diagnosis. Clin Cancer Res 2000;6:3994-9. [PubMed]
- Li H. Overview of The AJCC Lung Cancer Staging System, Eighth Edition may give us more thought: opportunities and challenges. Zhonghua Wai Ke Za Zhi 2017;55:346-50.
- Nakano N, Miyauchi K, Imagawa H, et al. Immediate localization using ultrasound-guided hookwire marking of peripheral lung tumors in the operating room. Interact Cardiovasc Thorac Surg 2004;3:104-6. [Crossref] [PubMed]
- Kleedehn M, Kim DH, Lee FT, et al. Preoperative Pulmonary Nodule Localization: A Comparison of Methylene Blue and Hookwire Techniques. AJR Am J Roentgenol 2016;207:1334-9. [Crossref] [PubMed]
- Long J, Petrov R, Haithcock B, et al. Electromagnetic Transthoracic Nodule Localization for Minimally Invasive Pulmonary Resection. Ann Thorac Surg 2019;108:1528-34. [Crossref] [PubMed]
- Wu CC, Maher MM, Shepard J-AO. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am J Roentgenol 2011;196:W678-82 [Crossref] [PubMed]
- Li M, Shen G, Gao F, et al. CT-guided fine-needle localization of ground-glass nodules in re-aerated lung specimens: localization of solitary small nodules or multiple nodules within the same lobe. Diagn Interv Radiol 2015;21:391-6. [Crossref] [PubMed]
- Chen W, Chen L, Qiang G, et al. Using an image-guided navigation system for localization of small pulmonary nodules before thoracoscopic surgery: a feasibility study. Surg Endosc 2007;21:1883-6. [Crossref] [PubMed]




