
EPAC-lung: European pooled analysis of the prognostic value of circulating tumour cells in small cell lung cancer
Introduction
Circulating tumour cells (CTCs) have been identified in a broad range of tumour types including lung cancer but are rarely seen in benign disease or healthy normal volunteers (HNV), thus making them an attractive biomarker (1). The current ‘gold standard’ method of CTC enumeration is the CellSearch® platform. CellSearch has been shown to be reliable and reproducible, with the FDA approving CellSearch CTC enumeration to inform on prognosis in metastatic breast, colorectal and prostate cancer (2-4). This efficient and semi-automated platform offers the opportunity for comparable large-scale studies with minimal inter user variation (5,6).
Small cell lung cancer (SCLC) characteristically presents with central rapidly growing tumours in which biopsies frequently harbour extensively necrotic tissue and scant tumour. Liquid biopsies offer an opportunity for systematic tumour interrogation, particularly important in this ‘recalcitrant’ cancer where emergence of chemo resistance is rapid, metastatic disease is early and prognosis is poor (7-9).
Despite its poor prognosis it is clinically evidence that SCLC patient outcomes are heterogeneous. A host of clinical and laboratory factors have been associated with poor outcomes in SCLC including performance status, age, sex, disease stage, LDH, albumin, creatinine, and sodium (10-17). Scoring systems that incorporate these details, such as the Manchester prognostic score, have been found to significantly associate with poorer survival (18). However, these have to some extent become obsolete as the guidelines for staging and care have updated, whilst efforts to upgrade prognostic scores often remain limited by the absence of pre-treatment variables recorded in large cancer databases (19,20). Identification of novel independent prognostic biomarkers that characterise patient subgroups remain important for prognostication and for stratifying patients in clinical trials.
An abundance of CTCs can be detected in the blood of patients with SCLC compared to other tumour types. Between 70–95% of patients with SCLC have detectable CTCs (21-30). Some relatively small single centre studies have aimed at evaluating the effect of the presence of CTCs on survival with some degree of discordance of prognostic results (22-24,26,29,31,32). This may be due to selection bias in the small patient series or a consequence of the semi-automated method of CTC enumeration, where CellSearch captures and identifies potential CTC candidates but ultimately individual trained users make the final decision on what represents a CTC.
Previous studies have identified thresholds of ≥2 and ≥50 CTCs as significant for inferior survival in heterogeneous cohorts of extensive and limited stage patients (22,27,31). The Phase III CONVERT study, which investigated once daily vs. twice daily chemoradiation in limited stage SCLC, found a threshold of 15 CTCs to be most significant for survival (32). These studies demonstrate that thresholds will vary according to the series studies and further consensus on the threshold, derived from a range of studies, would be required for clinical implementation.
This European cancer centre collaboration was established with the purpose to pool independent datasets for analysis of clinical associations and prognostic value of CTCs counts in SCLC. The primary outcome was to evaluate the relationship between pre-treatment CTC count and survival. Secondary analyses investigated inter site heterogeneity in CTC enumeration and the added value of incorporating CTCs into our clinic-pathological model. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-1061).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Gustave Roussy (Commission scientifique des Essais thérapeutiques) on July 20 2016. Centres were required to have local ethics committee approval for CTC enumeration and a recorded baseline CTC count prior to treatment for each individual and informed consent was taken from all individual participants.
Study design and population
The study protocol was designed by the study management team and reviewed by all investigators. Invitations to participate in the study were sent to 4 European Cancer Centres; known to treat SCLC patients and with the capabilities to enumerate CTCs with the CellSearch platform between Jan 2003 and March 2017. Eligible patients had a confirmed diagnosis of SCLC with available prospective or retrospective progression free survival (PFS) and overall survival (OS) data. Centres were required to have local ethics committee approval for CTC enumeration and a recorded baseline CTC count prior to treatment for each individual. Cases were excluded if CTC counts influenced clinical decision making by resulting in a treatment switch, thus avoiding confounding bias in the survival analysis.
Procedures
The Gustave Roussy cancer centre and the Cancer Research UK Manchester Institute (CRUK MI) partnered to establish the ‘EPAC-lung’ (European Pooled Analysis of CTCs in lung cancer) consortium. Other centres known to collect SCLC CTCs were then invited. Pseudo-anonymised patient data was collected, encrypted, and send to the central database by local investigators. The data included anonymised patient ID; centre ID; line of systemic treatment; baseline total CTC count by CellSearch (per 7.5 mL); CellSearch date; date of tumour progression and/or death; gender; age; ECOG performance status; smoking status; stage of disease (extensive vs. limited); planned treatment; and location/number of metastatic sites. Screening of data was performed by the study management team and any queries returned to the relevant centre.
Collection of blood, immuno-magnetic selection and immuno-fluorescent staining of CTCs were performed using the CellSearch® system, as previously described (6,33). All studies did not use the automated image analysis software ACCEPT, an open-source programme to identify CTC (https://github.com/LeonieZ/ACCEPT and www.cancer-id.eu).
Submitted data included CTCs counts previously published by participating centres (Figure 1) (24,26,31,32).

Statistical analysis
Study design and results are in accordance with recommendations for tumour markers (REMARK) criteria (34). Overall survival (OS) was defined as the time from first CTC analysis until death from any cause. Patients still alive were censored at the date of last follow-up. Progression free survival (PFS) was defined as time from first CTC analysis until confirmed tumour progression (as per assessment by RECIST 1.1 criteria) or death, whichever came first. If no event occurred, the record was censored at the date of last follow up.
The primary objective was to evaluate the prognostic effect of the quantitative amount of baseline pre-treatment CTC count (per 7.5 mL) by the CellSearch method in SCLC on OS and PFS. Analysis of CTCs as a continuous variable precluded the need for ROC curve analysis, although additional cut-offs of 15 and 50 were taken from previous single centre studies (31,32) in an effort to facilitate a standardised future approach to CTC adoption.
Associations between CTCs and survival were investigated using the Cox proportional hazard model and stratified by cancer centre. In order to investigate the linear relationship between CTCs and hazard of progression in the Cox regression model, cubic splines were used; a log-transformation was used in order to satisfy the linearity hypothesis. In addition to assessing CTCs as a continuous variable, pre-defined CTC thresholds were also included. Heterogeneity between centres was measured using chi-squared tests in the Cox regression models.
We prespecified a clinicopathological model for the multivariable Cox regression which included age (continuous), gender (male/female), baseline treatment (platinum doublet vs. other), smoking status (never smoked or former vs. current smoker), number of metastases (up to 1 vs. >1), performance status (ECOG score <2 vs. ECOG score ≥2) and sites of metastasis, then stratified by centre. Due to the low number of never smokers (3 patients) these were merged into the former smoker group for analysis. To assess the added value of CTCs to this clinico-pathological model in a multivariable Cox regression, we used likelihood ratios tests.
Associations between CTC counts and study population characteristics were analysed using Fishers exact test or Wilcoxon test. Kaplan Meier curves were used to estimate survival distributions. A two-sided significance level of 0.05 was considered significant.
Results
Four European cancer centres participated in the study, submitting pre-treatment CTC counts and survival data for 364 patients, of which 238 (65%) had extensive stage disease. The median pre-treatment CTC count was 19 with a range 0–44,896 CTCs detected. Two or more CTCs were detected in 266 (73%) patients of which 191 (53%) had ≥15 CTCs, and 139 (38%) had ≥50 CTCs counts of ≥15 and ≥50 was numerically higher for increased age, poorer performance status, extensive stage disease and increased number of metastasis. Table 1 displays the patient characteristics for the overall population and Table 2 patient characteristics divided by CTC cut-offs.
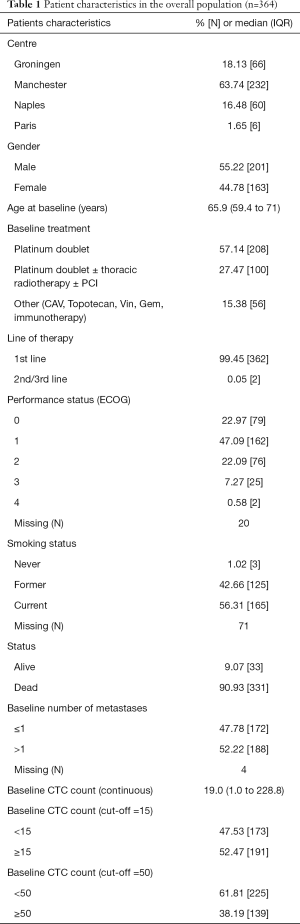
Full table
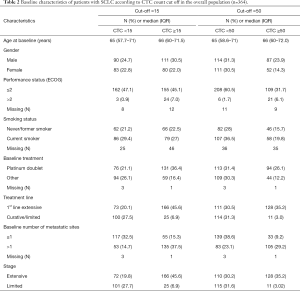
Full table
A total of 271 patients had sufficient clinical information available to be included in the multivariable analysis (see flow chart in Figure 1). Clinical data from one centre had to be excluded in the multivariate analysis as the patients smoking status was not recorded which was found to be clinically significant in the clinico-prognostic model.
Survival
The median follow-up for the pooled population was 62.4 months (95% CI: 46.3–68.9). The median PFS was 6.24 months (95% CI: 5.72–6.97) and median OS 7.85 months (95% CI: 6.93–8.87) at which time 338 patients had progressed and 331 patients died respectively.
For PFS, there was no significant heterogeneity observed between cancer centres for the prognostic effect of log transformed CTC counts (X32=3.12, P=0.37) or dichotomised CTC thresholds of ≥15 (X32=3.22, P=0.36), or ≥50 (X32=3.85, P=0.28) (Figure 2). In the primary analysis, a one-unit increase in log-transformed CTC counts corresponded to an estimated hazard ratio (HR) equal to 1.24 (95% CI: 1.19–1.29, P<2e-16). Using the cutoffs of 15 and 50 CTCs, a pre-treatment CTC count of ≥15 or ≥50 was significantly associated with an increased risk of progression (CTC ≥15 HR 3.20, 95% CI: 2.50–4.09, P<0.001, CTC ≥50 HR 2.56, 95% CI: 2.01–3.25, P<0.001) in univariable analysis (Figure 3A,B). The median PFS was 9.72 months (95% CI: 8.34–11.89) for <15 CTCs vs. 4.67 months (95% CI: 4.14–5.45) for ≥15 CTCs and median PFS for the higher CTC threshold <50 CTCs 7.75 months (95% CI: 7.03–9.46) vs. 4.57 months (95% CI: 3.75; 5.45) for ≥50 CTCs.
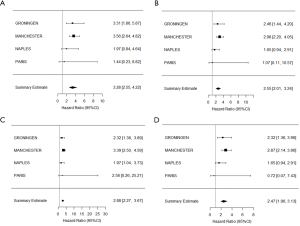
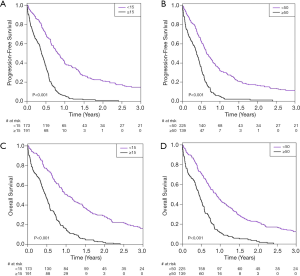
Regarding OS, no significant heterogeneity was observed between centres regarding the prognostic effect of CTCs for log-transformed CTCs (X32=2.60, P=0.457), nor CTC≥15 (X32=3.08, P=0.380), nor CTC ≥50 (X32=4.18, P=0.243) (Figure 2C,D). In the primary analysis, a one-unit increase in log-transformed CTC counts corresponded to an estimated hazard ratio (HR) equal to 1.23 (95% CI: 1.18–1.28, P<2e-16). Also, pre-treatment CTC counts of ≥15 was associated with an increased risk of death (OS HR 2.90, 95% CI: 2.28–3.70, P<0.001), as was pre-treatment CTC count ≥50 (OS HR 2.47, 95% CI: 1.95–3.13, P<0.001) (Figure 3C,D). The median OS for <15 CTCs was 12.30 months (95% CI: 10.50–16.00) vs. 5.65 months (95% CI: 4.76–6.44) for ≥15 and for <50 CTCs the median OS was 10.84 months (95% CI: 8.97-12.45) vs. 5.29 months (95% CI: 4.40–6.31) for ≥50 CTCs.
CTCs as an independent prognostic indicator
Prespecified clinico-pathological prognostic models were built incorporating identified prognostic factors, including age at baseline, gender, baseline treatment, performance status, smoking status, site of metastasis and number of metastasis.
The addition of log transformed CTC counts to clinico-pathological models resulted in a significant improvement in estimation of PFS (LR of 17.99, P=2.23e-05) and OS (LR 20.14, P=7.19e-06), confirming that CTC counts are an independent prognostic factor beyond established factors. Incorporating dichotomised CTC counts of ≥15 also yielded a significant LR for PFS (LR 15.36, P=8.89e-05) and OS (LR 13.35, P<0.001), while the higher threshold of >50 CTCs improved estimation of OS (LR 4.51, P=0.03) but not PFS (LR 2.65, P=0.103).
Discussion
In this European multicentre collaboration, we have confirmed that pre-treatment CTC count, enumerated by CellSearch, is an independent prognostic factor in SCLC. We observed minimal between-centre variability utilising this semi-automated enumeration platform.
Incorporation of CTC count, especially as a continuous variable, added value to our prespecified prognostic clinical-pathological model.
To our knowledge, this is the largest study to date evaluating the prognostic value of CellSearch CTC count in SCLC, and the only study that has analysed previous published and unpublished results from a number of European centres, thus addressing concerns regarding single centre heterogeneity. These findings support previous single centre reports (<100 patients), which have concluded that the presence of CTCs is associated with poor survival (23-26,29,31,32,35). Previous attempts at meta-analyses of the prognostic implications of CTCs in SCLC have yielded conflicting results, hampered by (I) selection bias through restriction to patients that have already been reported in published literature, (II) variability of CTC isolation platform employed for enumeration, and (III) univariable survival estimates only (30,36).
In this study, we observed only minimal heterogeneity in the association between CTC value and prognosis, supporting CellSearch as a standardised comparable platform for future studies. This result helps facilitate multi-site collaborations, dispelling any hypothetical concerns regarding the potential for inter-user inconsistency that may derive from image interpretation or lack of automated reporting software (37). This is particularly important as efforts are made to develop standards for CTC reporting across Europe through the CANCER-ID consortium (www.CANCER-ID.eu).
The limitations of our study include a residual potential for selection bias, incomplete data collection and the absence of a centralised pathological review. However, attempts to reduce bias have been made by large patient numbers and application of an established protocol in the limited number of centres performing CellSearch CTC quantification. A significant number of patient records were excluded from the study population due to incomplete data submission, including all data from one centre where smoking status could not be provided. The resulting study population incorporated published and unpublished data, supplemented by stratification according to cancer centre.
Our findings offer a definitive view of CTC prognostication in a cohort of limited and extensive stage SCLC. A previous large multicentre clinical trial, CONVERT, investigated the significance of baseline CTCs in a subset of 79 patients with limited SCLC, identifying a threshold of ≥15 CTCs as most strongly associated with poor survival (32). Other studies with a mix of limited and/or extensive stage SCLC patients have proposed numerous significant thresholds for prognosis (23,24,29,31). Our study has indicated that when using an appropriate log-transformation the effect of CTCs is pretty linear in a Cox regression model and that it is not a specific cut-off that drives prognosis.
Technology that isolates and/or enriches CTCs has evolved rapidly. Epitope dependent technologies such as CellSearch enrich for EpCAM expressing CTCs (33,38) whereas epitope independent systems e.g., Parsortix (39,40) and RosetteSep (41,42) exploit physical characteristics of CTCs to harvest cells independently of surface markers. RareCyte (43) and HD-SCA (44) can interrogate huge number of individual cells with the potential to identify rare CTC subpopulations. Discrete prognostic threshold for CTC enumeration will vary dependent upon CTC enrichment methodology and case series, favouring analysis of CTCs as a continuous variable.
Future work assessing longitudinal changes in CTC counts, in well powered studies, may also confirm CTCs as a surrogate for response and predictive for outcome, impacting clinical decision making. This study has confirmed the prognostic significance of baseline CTCs and would advocate incorporation of CTC counts into prognostic models and clinical trials, improving stratification of patients and trial design.
CTCs are already proving a hugely valuable resource in translation medicine. With established SCLC CTC derived xenografts (CDXs) (45) and the potential for SCLC CTC culture. Molecular characterisation of CTCs, employing a CNV classifier, has already proven to predict sensitivity to chemotherapy in extensive stage patients (46). As research into these clinically informative biomarkers increases, we have demonstrated the benefits of increased power and reduced bias from a collaborative approach of pooling multi-centre data.
Conclusions
In summary, this European collaboration has demonstrated that CTCs are an independent prognostic factor in SCLC. There was minimal inter site variability between European centres when utilising standardised CTC enumeration platforms, permitting pooled analysis of previously published and unpublished data. The continued pursuit of circulating biomarker research may soon yield more clinically applicable results which will establish their routine baseline and longitudinal use at critical junctures in patient care.
Acknowledgments
The authors would like to thank all the patients who took the time to participate in this research at a time when they had other priorities.
Funding: Dr. Lindsay was supported by the European Society for Medical Oncology (translational research fellowship – no grant number applicable) with the aid of a grant from Hoffman-La Roche and the International Association for the Study of Lung Cancer (no grant number applicable). CRL also received support as a recipient of the grant DUERTECC/EURONCO (Diplome Universitaire Européen de Recherche Translationelle et Clinique en Cancerologie - no grant number applicable). VF was funded via a Clinical Pharmacology Fellowship educational grant from CRUK and AstraZeneca (C147/A12328). The authors at the Gustave Roussy are grateful for the research support of the Fondation de France (grant no 201300038317), the Fondation ARC pour la Recherche sur le Cancer (grant no 20131200417), Innovative Medicines Initiative 11th Call CANCER ID (IMI-JU-11-2013, 115749), Institute National du Cancer (PRT-K14-032), Agence Nationale de la Recherche (ANR-CE17-0006-01) and the Ligue Contre Le Cancer (meta-analysis platform). This work was also supported by Cancer Research UK via funding to the CRUK Manchester Institute (Grant number A25254) and the CRUK Lung Cancer Centre of Excellence (Grant number A20465). Sample collection was undertaken as part of the CONVERT trial (Concurrent Once-daily Vs. twice daily RadioTherapy), and the ChemoRes trial (Molecular mechanisms underlying chemotherapy resistance, therapeutic escape, efficacy, and toxicity- Improving knowledge of treatment resistance in patients with lung cancer). This work was supported by the National Institute for Health research (NIHR) Manchester BRC, and the NIHR Christie Research Facility, and the Manchester MRC Single Cell Research Centre (MR/M008908/1). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. This project was also supported by funds from the Italian Ministry of Health through an intramural grant (M 2/1) of the Istituto Nazionale Tumori-IRCCS-Fondazione G. Pascale.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-1061
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-1061
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure forms (available at http://dx.doi.org/10.21037/tlcr-20-1061). Dr. CRL reports personal fees from Amgen, personal fees from copartners, outside the submitted work. Dr. CRL was supported by the European Society for Medical Oncology (translational research fellowship – no grant number applicable) with the aid of a grant from Hoffman-La Roche and the International Association for the Study of Lung Cancer (no grant number applicable). CRL also received support as a recipient of the grant DUERTECC/EURONCO (Diplome Universitaire Européen de Recherche Translationelle et Clinique en Cancerologie - no grant number applicable). Dr. VF was funded via a Clinical Pharmacology Fellowship educational grant from CRUK and AstraZeneca (C147/A12328). Dr. MGK reports personal fees from Janssen, personal fees from Roche, personal fees from Bayer, personal fees from Seattle Genetics, outside the submitted work. Dr. Groen reports grants from Cancer-ID project, outside the submitted work. Dr. BB reports grants from Abbvie, grants from Amgen, grants from AstraZeneca, grants from Biogen, grants from Blueprint Medicines, grants from BMS, grants from Celgène, grants from Eli-Lilly, grants from GSK, grants from Ignyta, grants from ISPEN, grants from Merck KGaA, grants from MSD, grants from Nektar, grants from Onxeo, grants from Pfizer, grants from Pharma Mar, grants from Sanofi, grants from Spectrum Pharmaceuticals, grants from Takeda, grants from Tiziana Pharma, outside the submitted work. Dr. AM reports personal fees from ROCHE, personal fees from ASTRAZENECA, personal fees from BOEHRINGER, from Pfizer, from BMS, from MSD, from TAKEDA, outside the submitted work. Dr. FP reports grants, personal fees and non-financial support from Bayer, personal fees from Sandoz, grants and personal fees from Incyte, personal fees from Celgene, grants and personal fees from Astra Zeneca, personal fees from Pierre Fabre, personal fees from Janssen Cilag, grants from Roche, grants from Pfizer, outside the submitted work. Dr. CFF reports grants and other from AstraZeneca, grants and other from Elekta, outside the submitted work. Dr. CD reports grants from AstraZeneca, grants from NIHR Manchester Biomedical Research Centre, during the conduct of the study; grants and personal fees from AstraZeneca, grants from Amgen, grants from Angle PLC, grants from Astex Pharmaceuticals, grants from Bayer, grants and personal fees from Biocartis, grants from Bioven, grants from BMS, grants from Boehringer Ingelheim, grants from Celgene, grants and non-financial support from Clearbridge Biomedics, grants from GSK, grants from Menarini Diagnostics, grants and personal fees from Merck KGaA, grants from Novartis, grants from Roche, grants from Taiho Oncology, non-financial support from Thermofisher, outside the submitted work. Dr. SM reports personal fees from Statistical advice: IDDI, Janssen CilagÐ personal fees from Independent Data Monitoring Committee member: Hexal, Steba, IQVIA, Roche, Sensorion, Biophytis, Servier, Yuhan, outside the submitted work. Dr. NN serves as an unpaid editorial board member of the Translational Lung Cancer Research from Sep 2019 to Sep 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board of Gustave Roussy (Commission scientifique des Essais thérapeutiques) on July 20 2016. Centres were required to have local ethics committee approval for CTC enumeration and a recorded baseline CTC count prior to treatment for each individual and informed consent was taken from all individual participants. Groningen- Patients were recruited, informed consent and samples obtained under the ethically approval of the Medical Ethical Committee (NTR5540). Naples: Patient recruitment, informed consent and sample collection was approved by Ethical Committee of the Pascale Institute (protocol n. 314/06). Manchester: Patients were recruited, informed consent and samples obtained under the ethically approved ChemoRes-Molecular mechanisms underlying chemotherapy resistance, therapeutic escape, efficacy and toxicity, ethics reference - 07/H1014/96) and CONVERT-A 2-Arm Randomized Controlled Trial of Concurrent Chemo-Radiotherapy Comparing Twice-Daily and Once-Daily Radiotherapy Schedules in Patients with Limited Stage Small Cell Lung Cancer (SCLC) and Good Performance Status - ethics reference 07/H1008/229) endorsed by the North West - Greater Manchester West Research Ethics Committee. Paris-This study was approved by the Institutional Review Board of Gustave Roussy (Commission scientifique des Essais thérapeutiques) on July 20 2016.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9. [Crossref] [PubMed]
- Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006;12:4218-24. [Crossref] [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. [Crossref] [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9. [Crossref] [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [Crossref] [PubMed]
- Riethdorf S, Fritsche H, Muller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13:920-8. [Crossref] [PubMed]
- . A plan of attack for deadly cancers. Cancer Discov 2014;4:980. [PubMed]
- Ujhazy P, Lindwasser OW. Small cell lung cancer: updates and new concepts. Transl Lung Cancer Res 2018;7:1-3. [Crossref] [PubMed]
- Levy B, Saxena A, Schneider BJ. Systemic therapy for small cell lung cancer. J Natl Compr Canc Netw 2013;11:780-7. [Crossref] [PubMed]
- Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group vs. International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer 2002;37:271-6. [Crossref] [PubMed]
- Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:300-11.
- Lassen U, Osterlind K, Hansen M, et al. Long-term survival in small-cell lung cancer: posttreatment characteristics in patients surviving 5 to 18+ years--an analysis of 1,714 consecutive patients. J Clin Oncol 1995;13:1215-20. [Crossref] [PubMed]
- Albain KS, Crowley JJ, LeBlanc M, et al. Determinants of improved outcome in small-cell lung cancer: an analysis of the 2,580-patient Southwest Oncology Group data base. J Clin Oncol 1990;8:1563-74. [Crossref] [PubMed]
- Rawson NS, Peto J. An overview of prognostic factors in small cell lung cancer. A report from the Subcommittee for the Management of Lung Cancer of the United Kingdom Coordinating Committee on Cancer Research. Br J Cancer 1990;61:597-604. [Crossref] [PubMed]
- Sculier J-P, Chansky K, Crowley JJ, et al. The Impact of Additional Prognostic Factors on Survival and their Relationship with the Anatomical Extent of Disease Expressed by the 6th Edition of the TNM Classification of Malignant Tumors and the Proposals for the 7th Edition. J Thorac Oncol2008;3:457-66.
- Souhami RL, Bradbury I, Geddes DM, et al. Prognostic significance of laboratory parameters measured at diagnosis in small cell carcinoma of the lung. Cancer Res 1985;45:2878-82. [PubMed]
- Wolf M, Holle R, Hans K, et al. Analysis of prognostic factors in 766 patients with small cell lung cancer (SCLC): the role of sex as a predictor for survival. Br J Cancer 1991;63:986-92. [Crossref] [PubMed]
- Cerny T, Anderson H, Bramwell V, et al. Pretreatment prognostic factors and scoring system in 407 small-cell lung cancer patients. Int J Cancer 1987;39:146-9. [Crossref] [PubMed]
- Wang S, Yang L, Ci B, et al. Development and Validation of a Nomogram Prognostic Model for SCLC Patients. J Thorac Oncol 2018;13:1338-48. [Crossref] [PubMed]
- Negre E, Coffy A, Langlais A, et al. Development and Validation of a Simplified Prognostic Score in SCLC. JTO Clin Res Rep 2020;1:100016 [Crossref]
- Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011;178:989-96. [Crossref] [PubMed]
- Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009;175:808-16. [Crossref] [PubMed]
- Naito T, Tanaka F, Ono A, et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J Thorac Oncol 2012;7:512-9. [Crossref] [PubMed]
- Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42. [Crossref] [PubMed]
- Huang CH, Wick JA, Sittampalam GS, et al. A multicenter pilot study examining the role of circulating tumor cells as a blood-based tumor marker in patients with extensive small-cell lung cancer. Front Oncol 2014;4:271. [Crossref] [PubMed]
- Normanno N, Rossi A, Morabito A, et al. Prognostic value of circulating tumor cells' reduction in patients with extensive small-cell lung cancer. Lung Cancer 2014;85:314-9. [Crossref] [PubMed]
- Igawa S, Gohda K, Fukui T, et al. Circulating tumor cells as a prognostic factor in patients with small cell lung cancer. Oncol Lett 2014;7:1469-73. [Crossref] [PubMed]
- Shi WL, Li J, Du YJ, et al. CK-19 mRNA-positive cells in peripheral blood predict treatment efficacy and survival in small-cell lung cancer patients. Med Oncol 2013;30:755. [Crossref] [PubMed]
- Cheng Y, Liu XQ, Fan Y, et al. Circulating tumor cell counts/change for outcome prediction in patients with extensive-stage small-cell lung cancer. Future Oncol 2016;12:789-99. [Crossref] [PubMed]
- Zhang J, Wang HT, Li BG. Prognostic significance of circulating tumor cells in small--cell lung cancer patients: a meta-analysis. Asian Pac J Cancer Prev 2014;15:8429-33. [Crossref] [PubMed]
- Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525-32. [Crossref] [PubMed]
- Tay RY, Fernández-Gutiérrez F, Foy V, et al. Prognostic value of circulating tumour cells in limited-stage small-cell lung cancer: analysis of the concurrent once-daily vs. twice-daily radiotherapy (CONVERT) randomised controlled trial. Ann Oncol 2019;30:1114-20. [Crossref] [PubMed]
- Kraan J, Sleijfer S, Strijbos MH, et al. External quality assurance of circulating tumor cell enumeration using the CellSearch((R)) system: a feasibility study. Cytometry B Clin Cytom 2011;80:112-8. [Crossref] [PubMed]
- Altman DG, McShane LM, Sauerbrei W, et al. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): explanation and elaboration. PLoS Med 2012;9:e1001216 [Crossref] [PubMed]
- Aggarwal C, Wang X, Ranganathan A, et al. Circulating tumor cells as a predictive biomarker in patients with small cell lung cancer undergoing chemotherapy. Lung Cancer 2017;112:118-25. [Crossref] [PubMed]
- Ma XL, Xiao ZL, Liu L, et al. Meta-analysis of circulating tumor cells as a prognostic marker in lung cancer. Asian Pac J Cancer Prev 2012;13:1137-44. [Crossref] [PubMed]
- Swennenhuis JF, van Dalum G, Zeune LL, et al. Improving the CellSearch(R) system. Expert Rev Mol Diagn 2016;16:1291-305. [Crossref] [PubMed]
- Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351:781-91. [Crossref] [PubMed]
- Xu L, Mao X, Imrali A, et al. Optimization and Evaluation of a Novel Size Based Circulating Tumor Cell Isolation System. PLoS One 2015;10:e0138032 [Crossref] [PubMed]
- Chudziak J, Burt DJ, Mohan S, et al. Clinical evaluation of a novel microfluidic device for epitope-independent enrichment of circulating tumour cells in patients with small cell lung cancer. Analyst 2016;141:669-78. [Crossref] [PubMed]
- Kulasinghe A, Perry C, Warkiani ME, et al. Short term ex-vivo expansion of circulating head and neck tumour cells. Oncotarget 2016;7:60101-9. [Crossref] [PubMed]
- Gao D, Vela I, Sboner A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014;159:176-87. [Crossref] [PubMed]
- Campton DE, Ramirez AB, Nordberg JJ, et al. High-recovery visual identification and single-cell retrieval of circulating tumor cells for genomic analysis using a dual-technology platform integrated with automated immunofluorescence staining. BMC Cancer 2015;15:360. [Crossref] [PubMed]
- Nieva J, Wendel M, Luttgen MS, et al. High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis. Phys Biol 2012;9:016004 [Crossref] [PubMed]
- Hodgkinson CL, Morrow CJ, Li Y, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med 2014;20:897-903. [Crossref] [PubMed]
- Carter L, Rothwell DG, Mesquita B, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med 2017;23:114-9. [Crossref] [PubMed]


