
A novel lung autotransplantation technique for treating central lung cancer: a case report
Introduction
Lung cancer, especially non-small cell lung cancer (NSCLC), is still the leading cause of cancer-related death in the world. Anatomically, lung cancer is divided into central and peripheral types. Some central lung cancer patients whose pulmonary function is insufficient to undergo pneumonectomy, will lose their opportunities for radical surgical treatment. The options for these patients are limited including chemotherapy, targeted therapy, immunotherapy with or without radiation. Some patients may have a second chance to receive surgical treatment after neo-adjuvant therapy (1), but the rest of them would lose their opportunities to receive radical surgery because of tumor progression or severe complications from chemotherapeutic medications.
Pneumonectomy for central lung cancer is associated with a high postoperative morbidity and mortality, especially with right-sided lesions. Although sleeve and double sleeve lobectomy offer satisfactory methods to treat central lung cancer and avoid pneumonectomy (2), those extensively involving the main bronchus or main pulmonary artery are very difficult to be resected using such techniques. In some cases, the surgical margin might have to be compromised in order to achieve tension-free anastomoses of pulmonary vessels and bronchus. Considering this, lung autotransplantation technique would be a suitable method to preserve lung tissue and completely remove the tumor (3-5). For the sake of simplifying the surgical process and widening the indication, we hereby report a novel lung autotransplantation technique. We present the following article in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-1242).
Case presentation
Case 1
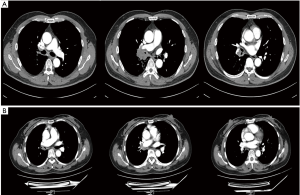
A 48-year-old male with a 30-pack-year smoking history and a family history of lung cancer (father) presented with paroxysmal irritative cough for 6 months. PET/CT scan showed: (I) a hypermetabolic right hilar mass invading right intermediate bronchus and right pulmonary artery trunk; (II) enlarged right hilar, carinal, and other mediastinal lymph nodes; (III) no sign of distant metastasis (Figure 1A). Bronchoscopic biopsy confirmed squamous cell lung cancer. Endobronchial Ultrasound (EBUS) confirmed negative 7 and 4R lymph nodes (cT3N0M0, IIB). PET/CT scan after 2 cycles of neo-adjuvant chemoimmunotherapy (paclitaxel + nivolumab) showed both the mass and mediastinal lymph nodes were slightly smaller and less hypermetabolic. Lung autotransplantation was considered to preserve the right basal segments.
Case 2
A 50-year-old female presented with progressive cough accompanied by dyspnea after exercise for 3 years. CXR showed a mass located in right hilum. No smoking or family history was reported. PET/CT showed: (I) irregularly thickened wall of the right main bronchus, intermediate bronchus and middle lobe bronchus with stenotic lumen. Atelectasis was observed in right middle lobe; (II) right pulmonary artery trunk was surrounded by the mass; (III) no enlarged or hypermetabolic mediastinal lymph node; (IV) no sign of distant metastasis (Figure 1B). Bronchoscopic biopsy indicated adenoid cystic carcinoma. EBUS showed the mediastinal lymph nodes were negative (cT3N0M0, IIB). Considering the extensive bronchial involvement from right main bronchus to middle lobe bronchus, right upper and middle lobe sleeve lobectomy with right lower lobe autotransplantation was proposed to preserve lung function and avoid pneumonectomy.
Surgical technique
After general anesthesia, the patient was placed on left lateral decubitus position. A 1 cm incision was made in the 7th intercostal space on the right anterior axillary line for the placement of thoracoscope. A 10 cm anterolateral incision in the 5th intercostal space for operation and a 1cm accessory incision in 7th intercostal space on right posterior axillary line were made. Right upper and middle lobectomy with S6 segmentectomy was firstly performed in Case 1. Right upper and middle lobectomy was performed in Case 2. The right intermediate bronchus was completely mobilized and the bronchial resection extended from the right main bronchus at carinal level to the orifice of graft bronchus. Frozen section of mediastinal lymph nodes and resection margins showed negative results. We therefore decided to perform right basal-segment and right lower lobe autotransplantation in Case 1 and Case 2, respectively.
After systemic heparinization (1 mg/kg), the pulmonary artery and vein of the graft were clamped and disconnected. Venous anastomosis was completed between right lower pulmonary vein of the graft and stump of right upper pulmonary vein. Right basal pulmonary artery was anastomosed to right upper lobe apical and anterior segment artery in Case 1. Pulmonary artery angioplasty without segmental resection was performed in Case 2. Vascular anastomoses were completed with continuous 5-0 Prolene.
In the reconstruction of airway, end-to-side anastomosis to the trachea was applied in both cases instead of end-to-end anastomosis to the right main bronchial stump. Lateral tracheal fenestration was performed above the level of carina. The tracheal opening was at least 1–1.5 cm2 in size and created entirely on the cartilaginous wall or partly including the edge between cartilaginous and membranous wall (6,7). The anastomosis between graft bronchus and lateral wall of trachea was started using parachute principle. A double-ended 3-0 Prolene was inserted from the cartilage wall of graft bronchus. The continuous sutures were firstly placed on the circumscribe of cartilage and tightened to turn the airway mucosa inward using nerve hooks. The remaining circumscribe of the membranous wall was sutured subsequently with the same suture to allow adjustment of anastomosis to avoid imbalance or excessive traction. The knots were tied on the outside of the airway (6). The anastomosis was then tested for air leaks up to 35 mmHg pressure. The anastomosis was finally covered with mediastinal pleural tissue circumferentially. The vessels and airway reconstruction procedures were diagrammatically demonstrated in Figures 2,3 and Video 1.

Hilar and mediastinal lymph node dissection was completed after reconstruction. Two chest tubes were inserted. The blood loss of Case 1 was 800 mL and the operation time was 358 minutes. They were 200 mL and 280 minutes in Case 2.
Postoperative management
Patient 1 was mechanically ventilated through endotracheal intubation on the first 2 postoperative days. Patient 2 was successfully extubated in the PACU after surgery. Both patients were closely monitored in the ICU. Prophylactic antibiotic and analgesics (remifentanil 0.03–0.01 µg/kg/min) were administered. Patients were not encouraged to cough in the first 3 days after operation. Bronchoscopy was performed daily to evaluate the anastomosis and remove secretion. Patients were transferred to normal wards when stable.
Postoperative short-term outcomes
Patient 1 was extubated on POD 2. Although bloody intratracheal secretion and sputum were observed after administration of LMWH for DVT prophylaxis, they gradually disappeared after discontinuing the anti-coagulation. He was transferred to normal ward on POD 4. Chest tubes were removed on POD 5 and 7 and he was discharged on POD 11. CXR before discharge indicated the graft was well-inflated without pleural effusion or pneumothorax.
Patient 2 was extubated in the post-anesthetic recovery room and given nasal cannula oxygen supply (1–2 L/min) in ICU. She was also administered LMWH for DVT prophylaxis. This resulted in increasing bloody drainage from the chest tubes and gradually lowering Hb which improved after discontinuing the LMWH and giving200 mL blood transfusion. She was transferred to normal ward on POD 4. Chest tubes were removed on POD 3 and 7 and she was discharged on POD 10. CXR before discharge showed no pleural effusion or pneumothorax.
Both cases underwent bronchoscopy before discharge. No anastomotic ischemia, tissue necrosis or dehiscence, was observed.
Pathological results
Pathological result of case 1 showed: (I) a 3.5 cm × 3 cm × 3 cm mass with hard texture, grey-white transection and irregular margin. Fibrinous tissue and massive lymphocyte infiltration were observed without viable tumor cell after neo-adjuvant chemotherapy; (II) all peri-bronchial, hilar and mediastinal lymph nodes were negative.
Pathological result of case 2 showed: (I) a 1.7 cm × 1.3 cm× 1 cm mass with hard texture, grey-white transection and regular margin. There were tumor cells in bronchial wall with fascicular invasions; (II) all peri-bronchial, hilar and mediastinal lymph nodes were negative. Immunohistochemistry showed P63(+), S-100(−), EMA(+), CK7(+), Ki-67(5%+). The final pathological diagnosis was bronchial adenoid cystic carcinoma.
Postoperative follow-up
Both patients underwent chest CT scan every 2 months and bronchoscopy every 3 months. Both patients complained of mild cough without any interference on their daily life at the first month follow-up. It could be alleviated by medications. No shortness of breath, chest pain, severe irritative cough or stridor was reported. Bronchoscopy showed no anastomotic stenosis or tumor recurrence at 9-month follow-up (Figure 4).

Ethics statement
The authors present the following article in accordance with the reporting checklist. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Lung cancer is the leading cause of cancer-related death in many countries. Unless there is distant metastasis, both early and locally advanced NSCLC could benefit from surgical resection in terms of oncological outcomes. However, central NSCLC are complicated and increase the difficulty of surgical treatment, especially when there is invasion of pulmonary vessels and/or main bronchus. Sleeve or double sleeve resections have become the primary choice of most thoracic surgeons to avoid pneumonectomy (8-11). But the limited length of the inferior pulmonary vein may jeopardize the anastomosis of pulmonary artery and/or bronchus. Pneumonectomy may be the only radical choice for these patients. However, its mortality was reported to be 4.9–8.0% (12-18). Significant loss of functional lung tissue might lead to poor quality of life. These conditions markedly limit the opportunity of patients who have compromised lung function. Lung autotransplantation may offer a suitable and feasible choice to achieve complete resection and functional lung tissue preservation. The most reported cases of lung autotransplantation are lower lobe or segments. When inferior pulmonary vein limited tension-free anastomosis of both bronchus and pulmonary artery anastomosis, reconnection of graft inferior pulmonary vein to the superior pulmonary venous stump is a feasible choice.
Two methods of lung autotransplantation have been reported which are ex situ and in situ. Some medical teams showed cases of local advanced lung cancer treating with en bloc pneumonectomy followed by ex situ separation of basal segment and perfusion with chilled glucose low-potassium dextran solution or UW solution (4,5,19). They believed this method is easier (2,5) and also reported that other surgeons could simultaneously finish the lymphadenectomy while the primary team was doing ex situ graft separation and perfusion on the back table and this would shorten the operation time (3). Some researchers reported cases of lung autotransplantation using in situ plastic reconstruction of bronchus, pulmonary artery, and pulmonary vein with satisfactory postoperative outcomes (20-22). They stated that it could shorten warm ischemic time since the graft was not prepared on the back table. In these cases, pneumonectomy was not performed but lobectomy with or without segmentectomy and subsequently followed by reconstruction.
There is still no consensus on whether graft perfusion is essential in both procedures. Oto et al. indicated that lung preservation with at least 2L of cold low-potassium phosphate-buffered solution was recommended when warm ischemic time exceeded one hour (23). We chose in situ method without graft perfusion when the following criteria were met: (I) the tumor could be resected and removed separately from the graft; (II) no complicated plastic reconstruction of the graft is needed; (III) reconstruction of vessels could be completed within 2 hours (24). We observed no ischemia/reperfusion-related short-term or long-term postoperative complication in either case.
Compared to Reardon’s case which used end-to-end bronchial reconstruction, we chose end-to-side method to reconnect graft bronchus to the lateral wall of trachea. Di Rienzo et al. previously reported the feasibility of this technique for bronchial reimplantation onto trachea or contralateral main bronchus in sleeve resections and carinal lobectomy (6). Adjusting the difference of diameter between main bronchus and graft bronchus is technically difficult and is associated with high tension (25). A tension-free anastomosis can be easily created by anastomosing the graft bronchus to the tracheal side wall. Furthermore, the diameter of fenestration created for anastomosis can be adjusted to the diameter of graft bronchus. The graft bronchus is reconnected to lateral wall of tracheal obliquely which also creates a larger lumen than end-to-end method. This type of anastomosis seems to use less suture than bronchoplasty and therefore might be easier and less time-consuming. In addition, end-to-end anastomosis is not possible when the main bronchus and/or the carina are invaded by tumor. In these situations, graft bronchus to trachea end-to-side anastomosis is the only method for airway reconstruction.
Additionally, it was reported that advancing the intermediate bronchus to the level of trachea for anastomosis can lead to airway necrosis and narrowing caused by anastomotic angulation, devascularization, and tension (26). Nevertheless, end-to-side anastomosis could be completed without excessive tracheal releasing maneuvers as end-to-end anastomosis, and might have less risk to impair tracheobronchial blood flow. Although end-to-side anastomosis to the contralateral main bronchus can be also considered as an alternative method, it might be more technically demanding and difficult because the anastomosis must be performed deeply among mediastinal structures such as the esophagus and pericardium (6,27). We therefore chose the trachea instead of the contralateral main bronchus to reconnect the graft bronchus. No tumor recurrence or anastomotic stenosis was observed during the 9-month postoperative follow-up. Postoperative anti-coagulation is critical to prevent DVT and pulmonary embolism. However, secondary bleeding may happen while using anti-coagulation medications.
In summary, we reported two cases of central NSCLC which were successfully treated with the novel lung autotransplantation technique. We used end-to-side graft bronchus to trachea anastomosis and in situ method to simplify the reconstruction. This technique might be easier and less time-consuming than the ex situ method. Neither short-term nor intermediate-term postoperative complication or recurrence was observed. Further study is warranted to elucidate the long-term survival and oncological outcomes of lung autotransplantation.
Acknowledgments
We thank Dr. Xin Xu, Dr. Guilin Peng and Dr. Hanzhang Chan from Department of Thoracic Surgery, Guangzhou Medical University First Affiliated Hospital for their help in the fields of surgical techniques and clinical practice.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-1242
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-1242
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-1242). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors present the following article in accordance with the reporting checklist. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bott MJ, Yang SC, Park BJ, et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:269-76. [Crossref] [PubMed]
- Okada M, Tsubota N, Yoshimura M, et al. Extended sleeve lobectomy for lung cancer: the avoidance of pneumonectomy. J Thorac Cardiovasc Surg 1999;118:710-3; discussion 713-4. [Crossref] [PubMed]
- Karube Y, Chida M, Nishihira M, et al. Back-table procedure and auto-lung transplantation for locally advanced lung cancer: a case report. J Cardiothorac Surg 2016;11:3. [Crossref] [PubMed]
- Oto T, Kiura K, Toyooka S, et al. Basal segmental auto-transplantation after pneumonectomy for advanced central lung cancer. Eur J Cardiothorac Surg 2012;42:579-81. [Crossref] [PubMed]
- Watanabe Y, Sato M, Nakamura Y, et al. Right lower lobe autotransplantation for locally advanced central lung cancer. Ann Thorac Surg 2015;99:323-6. [Crossref] [PubMed]
- Di Rienzo G, Go T, Macchiarini P. Simplified anastomotic technique for end-to-side bronchial reimplantation onto the trachea or contralateral main bronchus after complex tracheobronchial resections. J Thorac Cardiovasc Surg 2002;124:632-5. [Crossref] [PubMed]
- Costantino CL, Geller AD, Wright CD, et al. Carinal surgery: A single-institution experience spanning 2 decades. J Thorac Cardiovasc Surg 2019;157:2073-83.e1. [Crossref] [PubMed]
- Yoshino M, Saitoh Y, Chiyo M, et al. Surgical outcome of pulmonary artery reconstruction using the expanded polytetrafluoroethylene patch in patients with lung cancer. Surg Today 2019;49:778-84. [Crossref] [PubMed]
- Galetta D, Borri A, Gasparri R, et al. Surgical Techniques and Long-Term Results of Pulmonary Artery Reconstruction in Patients With Lung Cancer. Ann Thorac Surg 2015;100:1196-202; discussion 1202. [Crossref] [PubMed]
- Ma Q, Liu D, Guo Y, et al. Surgical techniques and results of the pulmonary artery reconstruction for patients with central non-small cell lung cancer. J Cardiothorac Surg 2013;8:219. [Crossref] [PubMed]
- Ricci C, Rendina EA, Venuta F, et al. Reconstruction of the pulmonary artery in patients with lung cancer. Ann Thorac Surg 1994;57:627-32; discussion 632-3. [Crossref] [PubMed]
- Green A, Hauge J, Iachina M, et al. The mortality after surgery in primary lung cancer: results from the Danish Lung Cancer Registry. Eur J Cardiothorac Surg 2016;49:589-94. [Crossref] [PubMed]
- Jakobsen E, Palshof T, Osterlind K, et al. Data from a national lung cancer registry contributes to improve outcome and quality of surgery: Danish results. Eur J Cardiothorac Surg 2009;35:348-52; discussion 352. [Crossref] [PubMed]
- Morgant MC, Pages PB, Orsini B, et al. Time trends in surgery for lung cancer in France from 2005 to 2012: a nationwide study. Eur Respir J 2015;46:1131-9. [Crossref] [PubMed]
- Powell HA, Tata LJ, Baldwin DR, et al. Early mortality after surgical resection for lung cancer: an analysis of the English National Lung cancer audit. Thorax 2013;68:826-34. [Crossref] [PubMed]
- Riaz SP, Linklater KM, Page R, et al. Recent trends in resection rates among non-small cell lung cancer patients in England. Thorax 2012;67:811-4. [Crossref] [PubMed]
- Seder CW, Raymond DP, Wright CD, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database 2017 Update on Outcomes and Quality. Ann Thorac Surg 2017;103:1378-83. [Crossref] [PubMed]
- Thomas PA, Berbis J, Baste JM, et al. Pneumonectomy for lung cancer: contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg 2015;149:73-82. [Crossref] [PubMed]
- Tang YJ, Wang CY, Dong YZ, et al. Lung autotransplantation for treating bronchus neoplasms. Chin Med J (Engl) 2007;120:2325-6. [Crossref] [PubMed]
- Jiang F, Xu L, Yuan FL, et al. Lung autotransplantation technique in the treatment for central lung cancer of upper lobe. J Thorac Oncol 2008;3:609-11. [Crossref] [PubMed]
- Mao W, Xia W, Chen J, et al. Successful lung autotransplantation for central non-small-cell lung cancer: report of a case. Surg Today 2013;43:562-5. [Crossref] [PubMed]
- Reardon MJ, Walkes JC, Rice DC. Autotransplantation for central non-small-cell lung cancer in a patient with poor pulmonary function. Tex Heart Inst J 2004;31:360-2. [PubMed]
- Oto T. Lung transplantation from donation after cardiac death (non-heart-beating) donors. Gen Thorac Cardiovasc Surg 2008;56:533-8. [Crossref] [PubMed]
- Levvey B, Keshavjee S, Cypel M, et al. Influence of lung donor agonal and warm ischemic times on early mortality: Analyses from the ISHLT DCD Lung Transplant Registry. J Heart Lung Transplant 2019;38:26-34. [Crossref] [PubMed]
- Yamamoto K, Miyamoto Y, Ohsumi A, et al. Surgical results of carinal reconstruction: an alterative technique for tumors involving the tracheal carina. Ann Thorac Surg 2007;84:216-20. [Crossref] [PubMed]
- Mitchell JD, Mathisen DJ, Wright CD, et al. Clinical experience with carinal resection. J Thorac Cardiovasc Surg 1999;117:39-52; discussion 53. [Crossref] [PubMed]
- Shiraishi T, Yamamoto L, Moroga T, et al. Transposition of pulmonary veins for mobilization of residual right middle and lower lobes after carinal right upper lobectomy: a unique pulmonary hilar mobilization technique for safe tension-free airway anastomosis. Gen Thorac Cardiovasc Surg 2020;68:1043-6. [Crossref] [PubMed]


