First line erlotinib for NSCLC patients not selected by EGFR mutation: keep carrying the TORCH or time to let the flame die?
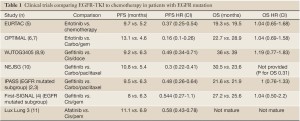
The BR.21 study evaluated the efficacy of erlotinib in an unselected population after progression on one or two prior chemotherapy regimens (1). The study demonstrated an improvement in median overall survival from 4.7 to 6.7 months, demonstrating the activity of erlotinib. Subsequently, the IPASS trial compared first line therapy with gefitinib or carboplatin-paclitaxel in the first line (2). Although the study did not select patients by molecular status, the study was conducted in East Asia and required patients to be never smokers or former light smokers. As a consequence, the study was biased towards patients with EGFR mutation; amongst patients with known mutation status, 60% had EGFR mutation. This subgroup seemed to drive the overall effect. Overall, the HR for PFS was 0.74, favoring gefitinib; amongst patients known to have EGFR mutation, the HR was 0.48. In contrast, for patients known to not have mutation, PFS was longer with chemotherapy, with HR 2.85. When final survival results were reported (3), the mOS was similar between gefitinib and chemotherapy for the overall study, for patients with EGFR mutation and for patients without mutation. This lack of survival difference was likely due to extensive crossover—patients who were initially treated with gefitinib received chemotherapy at the time of progression and patients who received chemotherapy initially received gefitinib at the time of progression. The results of First-SIGNAL (4), a trial of first line gefitinib vs. first line Gemcitabine and Cisplatin in Korean never-smokers with adenocarcinoma, were similar—a high rate of EGFR mutations (44%), a trend towards longer PFS in patients with EGFR-mutation (HR=0.54, P=0.086), and no significant differences in OS in either the overall study or in the EGFR-mutated subset (Table 1).
Full Table
These trials demonstrated superior disease control with TKI compared to chemotherapy. Therefore, for patients with EGFR mutation, 1st line therapy with a TKI is clearly appropriate. To many, the approach is even considered preferred in patients known to have EGFR mutation based on longer disease control and less side effects, even if ultimate survival is similar.
The TORCH study (12) re-asked this question in the context of an unselected population. Beyond the efficacy of erlotinib for an unselected population demonstrated in BR.21, two positive phase II studies provided more specific rationale for the first-line TORCH study. The first study treated 53 patients with erlotinib and resulted in a median survival of 391 days (13). Histologic tissue was available for molecular analysis in 29 patients; 7 EGFR mutations were found and survival in these patients trended towards superiority (627 vs. 377 days, P=0.15). 17 patients (32%) did not receive subsequent chemotherapy, although 4 received palliative radiotherapy. The second study (14) treated eighty elderly patients with erlotinib. Overall survival was 10.9 months. Survival was superior for patients with EGFR-mutation with a mOS of >15 months (n=9) compared to patients without mutation at 8.1 months (n=34, P=0.012). As in the previous study, histologic tissue was available for molecular in analysis for just over half of the patients and 56% did not receive subsequent chemotherapy. Positive results for unselected patients have also been reported for gefitinib (15,16). Thus, phase II studies laid a solid groundwork to justify phase III study of first-line erlotinib in unselected patients.
At the time of TORCH’s design, it was clear that both chemotherapy and EGFR-TKIs were active against NSCLC and the TKIs seemed to have fewer side effects for the average patient. In IPASS, the order of treatment did not affect survival, although the proportion of EGFR mutations was high. TORCH asked whether survival could be also preserved in a genuinely unselected population with the use of first-line erlotinib if crossover to chemotherapy were mandated at the time of progression. Patients with wet IIIB (now stage IV in the new staging system) or IV NSCLC and PS of 0-1 were randomized to first-line chemotherapy with cisplatin (80 mg/m2 day 1) plus gemcitabine 1,2000 mg/m2 (days 1, 8 of three week cycles) followed by erlotinib (150 mg QD) at progression or to erlotinib followed by chemotherapy at progression. Patients were stratified by histology, smoking status, sex, age, center and PS. Cross-sectional imaging to assess for progression was performed after 3 cycles and after 6 cycles of initial therapy, then every three months; the timing of radiologic assessment was the same for the two groups. Baseline characteristics of the patients, including histology and mutation status were balanced between the arms. Previous studies that did not formally select for EGFR mutation nonetheless biased their cohorts towards mutation by geography, histology, and by limiting the amount that patients could have smoked. In contrast, the inclusion criteria for TORCH did not favor patients with mutation. The mutation rate in IPASS was likely 60% because the study was conducted in Asia and was restricted to never or former light smokers. Similarly, in First-SIGNAL, patients were Korean, had adenocarcinoma, and were never-smokers; here, the mutation rate amongst patients with known mutation status was 44%. In contrast, the rate of EGFR mutation in TORCH was 14.2%, consistent with a truly unselected Western population.
Overall, the study failed to demonstrate non-inferiority of first-line erlotinib and the study was closed at first interim analysis. Median overall survival in the chemotherapy-first arm was 11.6 and 8.7 months in the experimental erlotinib-first arm (HR 1.24). The result held for all subgroups analyzed, including in both EGFR mutation negative and EGFR mutation positive patients.
The results for patients with EGFR mutation are unexpected. Although mPFS for EGFR mutated patients was numerically superior with 1st line erlotinib compared to 1st-line chemotherapy (9.7 vs. 6.9 months) their total PFS (time from treatment initiation until second progression) was actually numerically better with chemotherapy first (14.3 vs. 12.4 months) as was overall survival (32.5 vs. 18.1 months). These results are inconsistent with the larger literature (see table 1) and likely reflects the problem of small numbers. Overall, an absolute number of 39 patients who had known EGFR mutation were analyzed, 20 in the chemotherapy-first arm and 19 in the erlotinib-first arm. This results in a total number of events for survival of 24, for first progression of 36, and for second progression of 31. Therefore, first-line treatment with an EGFR-TKI remains a standard of care for patients known to have EGFR mutation.
TORCH, however, was not focused on patients with known EGFR-mutation, but rather on an unselected population. One great strength of the design was mandated crossover—TORCH asked a pure sequence question. The Fidias study of consolidation with docetaxel (17) suggested that exposure to an active agent might matter more than its timing. In this study, patients were treated with four cycles of gemcitabine and carboplatin before randomization to receive six cycles of consolidation docetaxel or to be observed and treated with docetaxel only at the time of progression. Overall, there was a trend towards superior survival with consolidation docetaxel (9.7 vs. 12.3 months, P=0.085). In this study, 40% of patients did not cross over, with the most common cause being disease progression. Among patients in the observation arm who did receive second line docetaxel at progression, survival was almost identical to patients on the consolidation arm (12.5 vs. 12.3 months). The differences in treatment efficacy as a result of sequencing in TORCH may also be driven more by failure to actually cross over than by timing per se. Indeed this seemed to be the case in TORCH. 42% of patients treated with erlotinib first failed to cross over to cisplatin plus gemcitabine. The primary reason, accounting for 70.5% of patients, was worsening/death, with refusal following at 11%. In contrast, only 29.5% of patients initially treated with chemotherapy failed to cross over to erlotinib. 62.2% here did not do so because of worsening/death and 7.8% because of refusal. Patients likely failed to cross over to chemotherapy after erlotinib because progression of disease left them too ill. Further, greater fear of chemotherapy side effects than erlotinib side effects may have driven the greater rate of patient refusal of second line therapy in the erlotinib-first group. Formal survival analysis of patients who did ultimately cross over would be helpful to understand whether differential crossover did indeed drive the survival differences seen.
Greater chemotherapy exposure in TORCH seemed to drive superior survival. This greater exposure also came at the cost of greater toxicity. Although rash and diarrhea were worse in the erlotinib-first arm, anemia, neutropenia, thrombocytopenia, fatigue, alopecia, nausea, and vomiting were all worse in the chemotherapy-first arm. Some patients prioritize avoidance of side effects over survival. However, less toxic therapy does not necessarily translate to superior quality of life when the more toxic therapy better controls cancer and thus cancer-related suffering. For example, the ELVIS study compared single-agent chemotherapy with vinorelbine to placebo for elderly patients (18). Although the vinorelbine arm did experience side effects, quality of life was superior because the vinorelbine prevented cancer-related suffering. In both TORCH and in the overall literature, chemotherapy controls lung cancer better for unselected populations than TKIs. If the incremental cancer-control with chemotherapy relieves more suffering than it creates vis-à-vis side effects, then the use of an erlotinib-first strategy to preserve quality of life in unselected patients, while intuitive, would be counterproductive. This may become even truer as advances in chemotherapy provide less toxic options and advances in supportive care reduce the toxicity of existing treatments. Quality of life during first treatment was assessed in TORCH utilizing the EORTC QLQ-C30 and the EORTC QLQ-LC13. Although the results were not reported in this initial publication, they could be useful for patient counseling.
In practice, oncologists do sometimes consider erlotinib for patients who are not candidates for chemotherapy, or who refuse it. The TOPICAL trial treated patients considered unfit for chemotherapy with erlotinib (19). To be eligible, patients needed to have a PS of 2-3 or PS1 with creatinine clearance <60 Survival with erlotinib was not superior to placebo. This suggests that for patients without EGFR mutation, who are not sufficiently fit for chemotherapy, that erlotinib may not represent a superior option than supportive care alone. This effect may not be restricted just to survival, but may also extend to quality of life.
The 8.7-month survival with erlotinib-first and 11.6-month survival with chemotherapy-first demonstrate the progress that has been made against NSCLC. Historically, patients with metastatic NSCLC not treated with chemotherapy lived about four months (20). When four platinum-based doublets were compared to each other in a study conducted between 1996 and 1999, survival was similar between the various regimens; in this study, survival with gemcitabine plus cisplatin was just under 8 months (21). Additional improvements leading to the 11.6 month survival with this same regimen in TORCH likely reflect the combination of better supportive care and the availability of superior subsequent therapies. This survival also exceeded the expectation of 10 months at trial design. Additional advances in treatment such as histology-directed therapy, knowledge of additional driver mutations, bevacizumab (for select patients), and maintenance therapy have further advanced survival.
Advances have also been made in molecular pathology. With modern methods, sensitivity for EGFR-detection may be rising (22). New methods promise to allow detection of EGFR-mutation from smaller samples, and perhaps even in blood (22). As a result, the frequency of unknown mutation status will likely decline and we may be entering an era where most NSCLCs are defined to be EGFR-positive or EGFR-negative. For patients whose cancers are known to be negative for EGFR-mutation or whose EGFR-mutation status is unknown, TORCH strongly supports the existing literature in favoring a chemotherapy-first approach.
Acknowledgements
Disclosure: Author received research funding from Astellas. Non-related sources of funding for research include GSK, Celgene, Acceleron and Pfizer.
References
- Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74.
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122-8.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42.
- Zhou C, Wu Y, Liu X, et al. Overall survival (OS) results from OPTIMAL (CTONG0802), a phase III trial of erlotinib (E) versus carboplatin plus gemcitabine (GC) as first-line treatment for Chinese patients with EGFR mutation-positive advanced non-small cell lung cancer (NSCLC). J Clin Oncol 2012;30:abstr 7520.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8.
- Mitsudomi T, Morita S, Yatabe Y, et al. Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol 2012;30:abstr 7521.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8.
- Yang JC, Schuler MH, Yamamoto N, et al. LUX-Lung 3: A randomized, open-label, phase III study of afatinib versus pemetrexed and cisplatin as first-line treatment for patients with advanced adenocarcinoma of the lung harboring EGFR-activating mutations. J Clin Oncol 2012;30:abstr LBA7500.
- Gridelli C, Ciardiello F, Gallo C, et al. First-Line Erlotinib Followed by Second-Line Cisplatin-Gemcitabine Chemotherapy in Advanced Non-Small-Cell Lung Cancer: The TORCH Randomized Trial. J Clin Oncol 2012;30:3002-11.
- Giaccone G, Gallegos Ruiz M, Le Chevalier T, et al. Erlotinib for frontline treatment of advanced non-small cell lung cancer: a phase II study. Clin Cancer Res 2006;12:6049-55.
- Jackman DM, Yeap BY, Lindeman NI, et al. Phase II clinical trial of chemotherapy-naive patients > or = 70 years of age treated with erlotinib for advanced non-small-cell lung cancer. J Clin Oncol 2007;25:760-6.
- Niho S, Kubota K, Goto K, et al. First-line single agent treatment with gefitinib in patients with advanced non-small-cell lung cancer: a phase II study. J Clin Oncol 2006;24:64-9.
- Yin YM, Geng YT, Shao YF, et al. First-line single agent treatment with gefitinib in patients with advanced non-small-cell lung cancer. J Exp Clin Cancer Res 2010;29:126.
- Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol 2009;27:591-8.
- Gridelli C. The ELVIS trial: a phase III study of single-agent vinorelbine as first-line treatment in elderly patients with advanced non-small cell lung cancer. Elderly Lung Cancer Vinorelbine Italian Study. Oncologist 2001;6:4-7.
- Lee S. Rudd R, Khan I, et al. TOPICAL: Randomized phase III trial of erlotinib compared with placebo in chemotherapy-naive patients with advanced non-small cell lung cancer (NSCLC) and unsuitable for first-line chemotherapy. J Clin Oncol 2010;28:abstr 7504.
- Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 1995;311:899-909.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8.
- Goto K, Satouchi M, Ishii G, et al. An evaluation study of EGFR mutation tests utilized for non-small-cell lung cancer in the diagnostic setting. Ann Oncol 2012. [Epub ahead of print].


