
Randomized phase III study comparing the first-line chemotherapy regimens in patients with driver mutation-negative advanced non-small cell lung cancer and poor performance status complicated with chronic obstructive pulmonary disease
Introduction
Lung cancer is one of the most common malignant tumors worldwide (1,2). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers, and approximately 75% of NSCLC is diagnosed at the advanced stage. For advanced NSCLC patients with good performance status (PS, 0–1), the standard treatment regimen is still mainly radiotherapy and chemotherapy. First-line targeted therapy is preferred for driver mutation-positive patients. Single or combined antiangiogenic therapy can also improve prognosis. Immune checkpoint therapy has gradually become a new treatment option in recent years (3). However, there are still 30% to 40% of advanced lung cancer patients with poor PS (2 points or above 2) only receiving supportive care without anti-tumor treatment (4). Most clinical trials exclude these patients due to their poor prognosis. To date, only a small number of studies have focused on patients with driver mutation-negative advanced NSCLC and poor PS complicated with chronic obstructive pulmonary disease (COPD), and found that chemotherapy is still recommend as the first-line treatment. Compared with single-drug chemotherapy and optimal supportive therapy, a number of clinical studies have confirmed that platinum-based dual-drug therapy, including pemetrexed (for adenocarcinoma) (5,6), paclitaxel (albumin) (7,8), and gemcitabine (9) can prolong survival in these patients, especially those with PS 2. Some studies have also confirmed that weekly low-dose chemotherapy can result in survival benefits and less adverse effects in advanced NSCLC patients with poor PS or in the elderly (10-13). However, there is no evidence for a preferred chemotherapy regimen for advanced NSCLC patients complicated with COPD and poor PS.
As a common concomitant disease of lung cancer, COPD is present in 40% to 70% of lung cancer patients (14,15). Lee et al. reported that 50.2% of 221 smoking patients with NSCLC had concomitant COPD (16), and Yi et al. (17) reported that 50.5% of 337 advanced NSCLC patients had COPD. Therefore, it has been proposed that the incidence of COPD among NSCLC patients is high (17). Compared with non-COPD-related NSCLC patients, patients with COPD have a lower mutation rate of EGFR and ALK (18). The article reported by Lim et al. (19) mainly focused on evaluating the effects of COPD on the overall survival of NSCLC patients undergoing conventional chemotherapy as the first-line treatment and indicated that COPD had a negative impact on the overall survival of the NSCLC patients in the smoker and stage IV subgroups. In terms of treatment, Omote et al. (20,21) suggested that NSCLC patients with mild to moderate or severe to extremely severe COPD can benefit from first-line chemotherapy, but the recommend regimens of chemotherapy are still unknown. In this study, we mainly focus on the special patients with driver mutation-negative advanced NSCLC and poor performance status complicated with COPD, the main purpose of this study is to analyze the efficacy and adverse effects of weekly low-dose regimens (docetaxel-carboplatin vs. gemcitabine-carboplatin) as the first-line chemotherapy regimen for these special patients, and explored the factors affecting the prognosis of patients.
We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-371).
Methods
Study design
This was a prospective, randomized, controlled, double-blind study, which was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University (No.2014A020212562) and registered on the Chinese Clinical Trial Registry website (CHiCTR-IPR-15006164). Written informed consent was obtained from all patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).This study enrolled patients with advanced NSCLC (excluding compound small cell lung cancer) newly diagnosed by cytology or histology at the First Affiliated Hospital of Guangzhou Medical University from January 1, 2015 to December 31, 2017, all that conformed to the criteria were included. The inclusion criteria were as follows: (I) treatment-naive NSCLC; (II) lung cancer patients at stage IIIb to IV who were evaluated to be unsuitable for surgery according to the 8th edition of the Union for International Cancer Control (UICC) staging criteria for NSCLC (22); (III) at least one measurable lesion was found according to the criteria for evaluating the efficacy of solid tumors (RECIST1.1); (IV) NSCLC patients had concomitant COPD, according to the diagnostic criteria of the Global Initiative on Chronic Obstructive Pulmonary Disease (GOLD) in 2019 (22), the forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) of patients after inhaling bronchodilators was less than 70% and the predicted value of FEV1% was less than 80% in pulmonary function tests, and imaging examinations supported the diagnosis of COPD; (V) EGFR, ALK, ROS1, BRAF, and KRAS mutations were detected by molecular biology methods (Burning Stone Biotechnology, Guangzhou, China) or EGFR/ALK mutations were detected by amplification block mutation system in polymerase chain reaction (PCR). Moreover, the negative of EGFR, ALK, ROS1, BRAF, and KRAS mutations were defined as driver mutation-negative in this study; (VI) PS ≥2; (VII) the estimated survival time was more than 3 months and at least 2 cycles of chemotherapy could be completed; (VIII) the performance status of lung cancer was based on the Zubrod-Eastern Cooperative Oncology Group-World health organization.
Before intervention, routine blood tests, liver and kidney function, electrocardiogram and other laboratory indexes of patients were basically normal, and the following criteria were met: (I) hemoglobin (Hb) ≥90 g/L (without blood transfusion in 14 days), absolute neutrophil count (ANC) ≥1.5×109/L, platelet (PLT) count ≥80×109/L; (II) bilirubin (BIL) <1.25 times normal upper limit (ULN), alanine transaminase (ALT) and aspartate aminotransferase (AST) <2.5 ULN. ALT and AST <5 ULN if there was liver metastasis. Serum creatinine (Cr) ≤1.25× ULN or endogenous Cr clearance >45 mL/min (Cockcroft-Gault formula). The exclusion criteria were as follows: known intolerance or allergies to experimental drugs or any excipient ingredient in these products; severe primary disease in vital organs or systems; patients with mental or physical disabilities; active stage of communicable diseases such as hepatitis A, hepatitis B, AIDS, tuberculosis, or connective tissue disease; patients with severe infections, especially pulmonary infections; patients participating in other clinical trials.
Treatment plan
The 55 patients were 1:1 randomly divided into two groups by a computer-generated random number table with a block size of two and sealed opaque envelope technique performed for allocation concealment by research designer, and they received weekly docetaxel 37.5 mg/m2, D1, D8 or gemcitabine 1,000 mg/m2, D1, D8, combined with carboplatin (AUC 5.0) D1 treatment, every 3 weeks, with planned treatment for 4–6 cycles. Neither the subjects nor the researchers were aware of the trial grouping. When adverse effects such as myelosuppression occurred during the process, related symptomatic treatment was given according to severity. All patients in this study were received the antiemetic medicine, proton pump inhibitors and liver protecting agents during the chemotherapy in order to reduce the occurrence of adverse reactions, which were classified according to National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE 4.03). The prophylactic administration of G-CSF was not permitted. Administration of IL-11 or TPO was permitted in patients with grade 2–3 thrombocytopenia and platelet transfusion was permitted in patients with grade 4 thrombocytopenia. All patients were treated for COPD according to the GOLD guidelines (23). For the patients who withdrew from the study after the first-line treatment progression or chose second-line and latter-line treatment according to the National Comprehensive Cancer Network (NCCN) guidelines and individual conditions, the study did not add intervention.
Evaluation of clinical efficacy and adverse effects
Before treatment, all patients underwent a complete medical history collection and physical examination, chest X-ray imaging, chest and abdomen computed tomography (CT) and enhancement, head CT or magnetic resonance imaging (MRI) scan and enhancement, bone scan, electrocardiogram, lung function test, and arterial blood gas analysis. Laboratory tests included complete blood count, liver function, serum electrolytes, kidney function, and stool and urine routine tests. Routine blood checks were performed 1–2 times a week, liver and kidney function and electrocardiograms were reviewed after each treatment cycle, and abdominal B-ultrasound and chest CT were reviewed after every 2 chemotherapy cycles for the evaluation of efficacy.
The clinical efficacy was evaluated according to RECIST 1.1 standards: complete response (CR) was defined as the disappearance of all measurable lesions, and partial response (PR) was defined as the sum of the maximum diameter of the baseline lesions reduced by at least 30%; stable disease (SD) meant that the sum of the maximum diameter of the baseline lesion was between PD and PR, and the minimum sum of diameters was used as a reference during the study; progressive disease (PD) was defined as the sum of the maximum diameter of the baseline lesion increasing more than 20%, or the appearance of new lesions; overall response rate (ORR) was defined as (CR + PR)/total number of cases ×100%; disease control rate (DCR) was defined as (CR + PR + SD)/total number of cases ×100%. The efficacy of treatment was evaluated every 2 cycles. Patients evaluated as CR, PR, and SD continued to the original plan, whereas the patients evaluated as PD withdrew from the trial and entered into second-line treatment (this study did not give intervention) until the patient died or the follow-up deadline was reached. Before PD, other anti-tumor treatments were not allowed. If the patient requested to stop the test before PD, the follow-up was performed every 2 months (approximately 56 days), and imaging examinations were performed to observe whether the tumor had progressed.
In this study, we evaluate the primary endpoints by progression-free survival (PFS), overall survival (OS). OS was the duration which was defined as the period from the time of diagnosis to the time of death for any cause, or was censored at the time to the last follow-up. The duration of PFS was defined as the period from the start of systemic therapy to the first date of disease progression or death, while median PFS (mPFS) was defined as the time to 50% of patients experiencing disease progression. Best support care (BSC), we took it as the period from the end of anti-tumor therapy to the end of life. The evaluation of adverse effects after chemotherapy was graded according to the evaluation criteria for common adverse events established by the National Cancer Center (CTCAE 4.03 version, http://ctep.cancer.gov).
Statistical analysis
Software SPSS 16.0 was used for the statistical analysis. Chi-square test or Fisher’s exact test was used to compare categorical variables, and a t-test or analysis of variance (ANOVA) was used to compare the differences between continuous variables. Survival analyses were estimated using Kaplan-Meier curves with the log-rank test. A two-sided P value less than 0.05 was considered statistically significant.
Results
Baseline characteristics of the patients
The detailed characteristics of 52 advanced NSCLC patients with COPD who had poor PS and were driver mutation-negative are shown in Table 1. All patients were diagnosed and treated in the First Affiliated Hospital of Guangzhou Medical University from January 1, 2015 to December 31, 2017. After randomization, 25 patients were enrolled in the docetaxel + carboplatin (DC) group, and 27 patients were enrolled in the gemcitabine + carboplatin (GC) group, and the Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Figure 1. There were no significant differences between the 2 groups in baseline characteristics including gender, age, body mass index (BMI), smoking status, pathological type, PS, TNM stage, metastasis site, and GOLD grade of lung function (P>0.05).
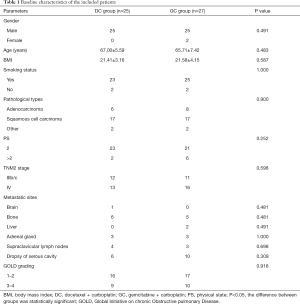
Full table
Analysis of clinical efficacy
In the DC group, the ORR and DCR were 20.0% and 72.0%, respectively, while in the GC group, the ORR was 22.2% and the DCR was 74.1%, and there was no significant difference between the 2 groups (Table 2). Median PFS (DC, 5.5 vs. GC, 6.5 months; P=0.296, Figure 2) and median OS (DC, 12.3 vs. GC, 14.9 months; P=0.548, Figure 2) also showed no statistical difference.

Full table

In the 52 patients in this study, 44 patients were treated with surface inhaled corticosteroid ICS) + long-acting β2 receptor agonist (LABA), 1 inhalation per time, twice per day. Eight patients were treated with inhalation of a long-acting anticholinergic drug (LAMA), 1 inhalation per time and once per day. We compared our study group to patients from a previous study by our research center (24) (Table 3), where 65 of 101 patients with advanced NSCLC complicated with COPD were only treated with anti-tumor therapy without treatment of COPD. Although PS was better than the patients in this group, there was no significant difference in tumor response rate including ORR and DCR. In addition, the median PFS in this study group was longer than that in the previous studies in the anti-tumor group alone (6.2 months, 95% CI: 3.533–6.733 vs. 3.5 months, 95% CI: 2.432–4.568; P=0.589), though the difference was not statistically significant. Furthermore, there was no significant difference in median OS between the 2 groups (14.0 months, 95% CI: 9.333–17.767 vs. 15.0 months, 95% CI: 8.606–21.394; P=0.718).
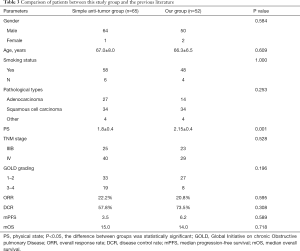
Full table
Adverse effects after first-line chemotherapy and its influence on second-line treatment
Among the 25 patients in the DC chemotherapy group, the most common adverse effect of chemotherapy was myelosuppression, among which 17 patients (68.0%) had grade 1–2 myelosuppression, while grade 3–4 myelosuppression occurred in 3 patients (12.0%). Myelosuppression was also the most common adverse effect in the GC group. A total of 15 patients (53.6%) had grade 1–2 myelosuppression, and 2 patients (7.0%) had grade 3–4 myelosuppression. The above adverse effects were alleviated after symptomatic treatment, and all patients were able to tolerate later treatment without serious adverse effects (Table 4).

Full table
After the disease progression of first-line treatment, a total of 35 patients entered second-line treatment, and the number of patients in the DC group who entered second-line treatment was greater than that of the GC group (80% vs. 55.6%, P=0.060). There was no statistically significant difference in second-line treatment cycles between the 2 groups. The second-line treatment was still dominated by chemotherapy (62.9%), and the adverse effects after second-line treatment were still dominated by myelosuppression (37.1%). However, the number of grade 3–4 adverse effects was less, accounting for only 8.6%, and the adverse effects of other systems were relatively lower (Table 5).

Full table
The prognosis of survival
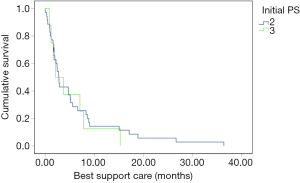
The follow-up period was up until April 30, 2020, and the median follow-up period was 12.3 months (3.3–46.7 months). Of the 52 patients, 3 were lost to follow-up, 43 died and 6 are still alive. According to the initial PS, patients who died at the last follow-up were divided into two groups: initial PS =2 group (n=35) and initial PS =3 group (n=8). The median estimated BSC was 2.80 and 2.10 months, respectively. The BSC survival curves of patients with different initial PS scores were crossed (log rank P=0.686, Figure 3).
The median PFS of patients was 6.0 months, and the PFS rate in 0.5 and 1 year was 40% and 15%, respectively (Figure 4). The median OS was 14.0 months, and the 1- and 2-year OS rates were 52% and 28%, respectively (Figure 4). In the single-factor analysis, pathological type of tumor (P<0.001; P=0.014, Figure 5), TNM stage (P=0.047; P=0.011, Figure 6), and the cycle of chemotherapy (P<0.001; P=0.006, Figure 7) were prognostic factors for PFS and OS. And multivariate analysis was performed to remove confounders. In the multivariate analysis, chemotherapy cycle (P<0.001) was the independent factor for PFS, while chemotherapy cycle (P=0.011) and PS (P=0.041) were independent factors for OS (Table 6).




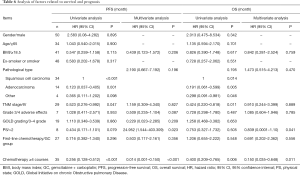
Full table
Discussion
Although driver mutation-negative advanced NSCLC patients with poor PS are common, their treatment and whole-course management is difficult due to their poor tolerance to cytotoxic drugs. Present studies have found that these special populations could benefit from single-agent or platinum-based chemotherapy (13,25-27) compared with supportive care only, and more and more attention is being paid to explore comparable chemotherapy regimens. In this study, driver mutation-negative advanced NSCLC patients with poor PS complicated with COPD were selected as the target population, and the clinical efficacy and safety of low-dose platinum-based chemotherapy, namely, a weekly low-dose regimen of carboplatin-based docetaxel as the first-line chemotherapy regimen, were analyzed prospectively compared with carboplatin-based gemcitabine. The results of this study showed that this special population could benefit from these drugs as well as tolerate their toxicity, and there was no significant difference in terms of clinical efficacy and safety between these 2 groups.
Most of the patients in this study were elderly male smoking patients, and the majority of pathological types were squamous cell lung cancer, which was similar to a report from Lim et al. (19). They reported 149 advanced NSCLC patients with a mean age of 69.5±9.4 years, where 85.9% of patients were males, 81.5% of them were smokers, and 50.0% of them had squamous cell lung cancer. This phenomenon may be explained by the fact that these 2 diseases are closely related to smoking, especially squamous cell lung cancer, which has a closer correlation with smoking. Furthermore, the incidence of COPD complicated with lung cancer increases year by year after the age of 40, and reaches a peak at 60 (28), which suggests that most of the patients in this group are elderly with poor PS, making the treatment regimen for this group extremely challenging in clinical practice. Therefore, it is generally believed that patients with driver-mutation negative advanced NSCLC complicated with COPD, especially those with poor PS, cannot tolerate cytotoxic drug therapy and should be only given supportive care. Lim et al. (29) found that longer OS was related to aggressive anti-tumor therapy. The mild to moderate COPD reported by Omote et al. (20) had no effect on the toxicity and prognosis of NSCLC patients treated with chemotherapy. Furthermore, Dong et al. (21) performed a study on 267 NSCLC patients with severe to extreme COPD, 54 of them receiving chemotherapy and supportive care, 24 of them only receiving supportive care, and they found that the median OS of patients in the chemotherapy group was 6 months longer than patients in the non-chemotherapy group (14.0 months, 95% CI: 8.5–19.5 vs. 8.0 months, 95% CI: 6.4–9.6. P=0.003), while the incidence rate of grade 3–4 adverse effects was 29%. Therefore, it suggest that patients with advanced NSCLC combined with COPD may benefit from chemotherapy.
Qin et al. (30,31) found that inhalation of ICS could improve the PS and prolong PFS of the lung cancer patients complicated with COPD. Meanwhile, Sekine et al. (32) also pointed out that non-standardized treatment of COPD not only reduce their quality of life and increase the frequency of acute exacerbation of COPD, but also have an influence on the long-term effect of anti-tumor treatment. Therefore, Young et al. (33) proposed that lung cancer patients complicated with COPD should receive multidisciplinary treatment. To our knowledge, previous studies involving lung cancer complicated with COPD mainly focused on the efficiency and (or) safety of anti-tumor therapy and the impact of COPD on the prognosis, but few of them emphasize on the treatment of COPD. Hence, we designed our study by including 52 patients who used combined ICS and LABA with or without LAMA to control the symptoms of COPD according to the GOLD guidelines along with the whole process of anti-tumor treatment, and compared with a previous retrospective study from our center which reported on 65 driver mutation-negative advanced NSCLC patients with COPD only receiving anti-tumor drug therapy (24). Although patients in our study had worse PS, the median PFS in our group was longer compared to patients only receiving anti-tumor therapy, though this did not reach statistical significance. Furthermore, the median OS and tumor response rate including ORR and DCR demonstrated no significant differences between groups, which was similar to Wang et al. (24). This suggests that patients in our study improved their PS by controlling the symptoms of COPD, which further improved their prognosis. Meanwhile, Wang et al. (24) also found that most of these patients were in the acute exacerbation stage of COPD at the first diagnosis of lung cancer, with poor PS (above 2), which could be significantly improved by reasonable control of COPD symptoms, and the median OS and PFS were superior to the anti-tumor therapy only group (24). Therefore, this study emphasizes that in the co-treatment of lung cancer, attention should be paid to the routine treatment of concomitant basic lung diseases, keeping in mind the reversibility and volatility of PS score.
According to the OPTIMAL trial (34) and the TIMELY trial (35), the application of EGFR-tyrosine kinase inhibitors (TKIs) in patients with advanced driver mutation- positive NSCLC with PS 2–3 could prolong their median PFS and OS. However, those with driver mutation-negative NSCLC lost the opportunity for EGFR-TKI treatment in this study. In recent years, more and more clinical trials have shown that compared with supportive care or single drug treatment, NSCLC patients with poor PS can still benefit more from platinum-based chemotherapy and tolerate its adverse effects (7,25,36). In contrast to the traditional 3-week chemotherapy regimen, low-dose injection in batches and lengthening the time could increase the full exposure of the tumor to chemotherapy drugs, which maybe improve the treatment efficacy and decrease adverse effects, ultimately improving the compliance and tolerance of chemotherapy. Furthermore, increasing the number of medications is more suitable for the elderly or patients with poor PS (10,37,38). Studies have also found that for weekly low-dose regimens including docetaxel (10,11,38), paclitaxel (13,27), and gemcitabine (12,36) compared with the standard 3 week regimen, the incidence of grade 3–4 adverse effects was also lower, especially in elderly patients or those with poor PS.
The ORR and DCR of the 2 groups of patients in this study were good, which is consistent with the tumor response rate of docetaxel combined with cisplatin in the first-line treatment of advanced NSCLC in several phase II and phase III clinical trials worldwide, which ranged between 20–39% (39). The ORR, DCR, median PFS, and median OS of patients in the GC group were better than the DC group, but the difference was not statistically significant. These results are similar to the results of a randomized multicenter phase II study of weekly docetaxel/cisplatin (DP, n=50 cases) and gemcitabine/cisplatin (GP, n=47 cases) as first-line treatment for elderly or advanced NSCLC patients with poor PS conducted by Jang et al. (12). Gao et al. (40) also reported 43 patients with advanced NSCLC who were treated with DP or GP as the first-line treatment. The results showed that there was no significant difference in tumor response rate and median survival time between the 2 groups, and adverse effects were tolerable. The ECOG1594 study compared 4 chemotherapy regimens including gemcitabine, docetaxel, and paclitaxel combined with cisplatin or carboplatin in the treatment of advanced NSCLC. This study also showed that there was no significant difference in the efficacy of the 4 chemotherapy regimens. The PFS and OS of gemcitabine were longer than those of the other groups, especially in patients with squamous cell carcinoma (41). Therefore, patients in the gemcitabine group seem to have a better survival benefit in this study, which may be related to the majority of patients with squamous cell carcinoma in this study. In addition, there were more cases in the DC group entering second-line treatment. Does this suggest that the DC regimen may be better tolerated and more conducive to follow-up treatment in order to achieve whole-course management? Further studies with larger sample sizes are required for confirmation. Studies have suggested that COPD predicts worse OS in NSCLC patients who smoke (42). This study found that the number of chemotherapy cycles is an independent influencing factor of PFS and OS, the potential reason being that the possibility of tumor control may increase with the number of treatment cycles. Weekly low-dose chemotherapy regimens have this advantage. A phase II clinical trial showed that a weekly low-dose DP regimen (docetaxel 20 mg/m2 + cisplatin 25 mg/m2, D1, D8, D15) used in patients with advanced NSCLC aged ≥75 years obtained better tumor response rates, with DCR reaching 52% and median OS reaching 15.8 months (43). Therefore, whether it is a weekly low-dose plan for 2 or 3 weeks, this regimen is suitable for elderly patients with poor PS, which not only increases chemotherapy tolerance and reduces adverse effects, but also improves the curative effect and prognosis. However, the impact on patients’ quality of life and economic burden needs to be assessed. This study also showed that PS is an independent influencing factor of OS. Stinchcombe et al. (44) found that in advanced NSCLC patients who underwent carboplatin-based chemotherapy, though PS had no effect on tumor response rate, it was associated with a worse prognosis (median OS, 4.9 vs. 8.4 months, P=0.0004). Therefore, poor PS predicts the prognosis of patients, and improving their PS can produce better treatment tolerance and prognosis.
This study was a prospective study carried out a few years ago, and has some limitations. Firstly, the sample size of this study is limited, and follow-up still requires a larger sample size and a prolonged time for further confirmation. Secondly, this study did not evaluate the quality of life of patients, and no relevant research has been done.
Overall, this study shows that although the treatment of patients with driver mutation-negative advanced NSCLC and poor PS complicated with COPD is difficult, we should keep in mind the concept and treatment principles of “severe lung cancer” proposed by our team in the early stage (45,46), and pay attention to the reversibility and volatility of PS. Moreover, we should also comply with the principles of simultaneous treatment of cancer and lung diseases, and whole-course management. The use of weekly low-dose docetaxel or gemcitabine combined with carboplatin chemotherapy can achieve survival benefits and safety, however, there is no significant difference in curative effect and safety between these 2 regimens.
Acknowledgments
The authors appreciate the academic support from AME Lung Cancer Collaborative Group.
Funding: This study was funded by Clinical application and translational research projects of the First Affiliated Hospital of Guangzhou Medical University (201510-gyfyy), High Level University Academic Backbone Cultivation Plan of Guangzhou Medical University Established (201721020), Characteristic innovation projects in ordinary universities in Guangdong Province, Mandatory project of Guangdong Medical Research Fund (2018KTSCX181), Mandatory subject project of medical research fund of Guangdong province (C2019029)/Self-funded science and technology plan project of Guangdong department of science and technology (2019ZC0029), General Program of the State Key Laboratory of Respiratory Diseases (SKLRD-MS-201905), and New Coronary Pneumonia Scientific Research Project of Guangdong Provincial Department of Education in 2020 (2020KZDZX1162).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-371
Trial Protocol: Available at https://dx.doi.org/10.21037/tlcr-21-371
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-371
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-371). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study originated from a Chinese clinical trial registry project, a Guangdong province science and technology plan project, and it was conducted with approval from the Ethics Committee of The First Affiliated Hospital of Guangzhou Medical University, the project contract no. 2014A020212562. Written informed consent was obtained from all patients. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Li DH, Yao Y, Geng Q. Interpretation of the update of the guideline for the comprehensive diagnosis and treatment of liver cancer (2018 edition). Journal of Clinical Surgery 2019;27:36-9.
- Langer CJ. Clinical evidence on the undertreatment of older and poor performance patients who have advanced non-small-cell lung cancer: is there a role for targeted therapy in these cohorts? Clin Lung Cancer 2011;12:272-9. [Crossref] [PubMed]
- Schluckebier L, Garay OU, Zukin M, et al. Carboplatin plus pemetrexed offers superior cost-effectiveness compared to pemetrexed in patients with advanced non-small cell lung cancer and performance status 2. Lung Cancer 2015;89:274-9. [Crossref] [PubMed]
- Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013;31:2849-53. [Crossref] [PubMed]
- Gajra A, Karim NA, Mulford DA, et al. nab-Paclitaxel-Based Therapy in Underserved Patient Populations: The ABOUND.PS2 Study in Patients With NSCLC and a Performance Status of 2. Front Oncol 2018;8:253. [Crossref] [PubMed]
- Lilenbaum RC, Herndon JE 2nd, List MA, et al. Single-agent versus combination chemotherapy in advanced non-small-cell lung cancer: the cancer and leukemia group B (study 9730). J Clin Oncol 2005;23:190-6. [Crossref] [PubMed]
- Morabito A, Gebbia V, Di Maio M, et al. Randomized phase III trial of gemcitabine and cisplatin vs. gemcitabine alone in patients with advanced non-small cell lung cancer and a performance status of 2: the CAPPA-2 study. Lung Cancer 2013;81:77-83. [Crossref] [PubMed]
- Lee KW, Lim JH, Kim JH, et al. Weekly low-dose docetaxel for salvage chemotherapy in pretreated elderly or poor performance status patients with non-small cell lung cancer. J Korean Med Sci 2008;23:992-8. [Crossref] [PubMed]
- LeCaer H, Barlesi F, Robinet G, et al. An open multicenter phase II trial of weekly docetaxel for advanced-stage non-small-cell lung cancer in elderly patients with significant comorbidity and/or poor performance status: The GFPC 02-02b study. Lung Cancer 2007;57:72-8. [Crossref] [PubMed]
- Jang J, Kim HK, Cho BC, et al. Randomized phase II study comparing weekly docetaxel-cisplatin vs. gemcitabine-cisplatin in elderly or poor performance status patients with advanced non-small cell lung cancer. Cancer Chemother Pharmacol 2017;79:873-80. [Crossref] [PubMed]
- Sakakibara T, Inoue A, Sugawara S, et al. Randomized phase II trial of weekly paclitaxel combined with carboplatin versus standard paclitaxel combined with carboplatin for elderly patients with advanced non-small-cell lung cancer. Ann Oncol 2010;21:795-9. [Crossref] [PubMed]
- Loganathan RS, Stover DE, Shi W, et al. Prevalence of COPD in women compared to men around the time of diagnosis of primary lung cancer. Chest 2006;129:1305-12. [Crossref] [PubMed]
- Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 2007;370:741-50. [Crossref] [PubMed]
- Lee SJ, Lee J, Park YS, et al. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol 2014;9:812-7. [Crossref] [PubMed]
- Yi YS, Ban WH, Sohng KY. Effect of COPD on symptoms, quality of life and prognosis in patients with advanced non-small cell lung cancer. BMC Cancer 2018;18:1053. [Crossref] [PubMed]
- Lim JU, Yeo CD, Rhee CK, et al. Chronic Obstructive Pulmonary Disease-Related Non-Small-Cell Lung Cancer Exhibits a Low Prevalence of EGFR and ALK Driver Mutations. PLoS One 2015;10:e0142306 [Crossref] [PubMed]
- Lim JU, Yeo CD, Rhee CK, et al. Overall survival of driver mutation-negative non-small cell lung cancer patients with COPD under chemotherapy compared to non-COPD non-small cell lung cancer patients. Int J Chron Obstruct Pulmon Dis 2018;13:2139-46. [Crossref] [PubMed]
- Omote N, Hashimoto N, Morise M, et al. Impact of mild to moderate COPD on feasibility and prognosis in non-small cell lung cancer patients who received chemotherapy. Int J Chron Obstruct Pulmon Dis 2017;12:3541-7. [Crossref] [PubMed]
- Dong W, Du Y, Ma S. Impact of chemotherapy in the prognosis of non-small-cell lung cancer patients with severe to very severe COPD. Int J Chron Obstruct Pulmon Dis 2018;13:3805-12. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J 2019; [Crossref] [PubMed]
- Wang F, Xie XH, Lin XQ, et al. Exploration of the treatment model for patients with advanced non-small cell lung cancer complicated with chronic obstructive pulmonary disease based on real-world data. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:450-4. [PubMed]
- Zinner R, Visseren-Grul C, Spigel DR, et al. Pemetrexed clinical studies in performance status 2 patients with non-small cell lung cancer Int J Oncol 2016;48:13-27. (Review). [Crossref] [PubMed]
- Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy and supportive care versus supportive care alone for advanced non-small cell lung cancer. Cochrane Database Syst Rev 2010;CD007309 [PubMed]
- Liu L, Wang XW, Li L, et al. A randomized comparative trial of three combined regimens containing cisplatin for treatment of advanced non-small cell lung cancer. Ai Zheng 2006;25:990-4. [PubMed]
- Kiri VA, Soriano J, Visick G, et al. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J 2010;19:57-61. [Crossref] [PubMed]
- Lim JU, Yeo CD, Rhee CK, et al. Comparison of clinical characteristics and overall survival between spirometrically diagnosed chronic obstructive pulmonary disease (COPD) and non-COPD never-smoking stage I-IV non-small cell lung cancer patients. Int J Chron Obstruct Pulmon Dis 2019;14:929-38. [Crossref] [PubMed]
- Qin YY, Zhou CZ, Zhang XX, et al. Clinical study of patients with primary bronchogenic lung cancer complicated with chronic obstructive pulmonary disease. Chinese Journal of Respiratory and Critical Care Medicine 2013;12:65-8.
- Miller YE, Keith RL. Inhaled corticosteroids and lung cancer chemoprevention. Am J Respir Crit Care Med 2007;175:636-7. [Crossref] [PubMed]
- Sekine Y, Yamada Y, Chiyo M, et al. Association of chronic obstructive pulmonary disease and tumor recurrence in patients with stage IA lung cancer after complete resection. Ann Thorac Surg 2007;84:946-50. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J 2009;34:380-6. [Crossref] [PubMed]
- Lee SM, Khan I, Upadhyay S, et al. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2012;13:1161-70. [Crossref] [PubMed]
- Sanjay P, Hughes L, O'Brien M, et al. P3.02b-046 Afatinib Benefits Patients with Confirmed/Suspected EGFR Mutant NSCLC, Unsuitable for Chemotherapy (TIMELY Phase II Trial). J Thorac Oncol 2017;12:S1215-16. [Crossref]
- Frasci G, Lorusso V, Panza N, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 2000;18:2529-36. [Crossref] [PubMed]
- Kerbel RS, Klement G, Pritchard KI, et al. Continuous low-dose anti-angiogenic/ metronomic chemotherapy: from the research laboratory into the oncology clinic. Ann Oncol 2002;13:12-5. [Crossref] [PubMed]
- Schuette W, Nagel S, Blankenburg T, et al. Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol 2005;23:8389-95. [Crossref] [PubMed]
- Zhong X. Cisplatin Plus Docetaxel Combination in the First-line Treatment of Advanced Non-small Cell Lung Cancer. Zhejiang University, 2011.
- Gao Y, Shi ZQ, Cao CW, et al. A randomized trial of docetaxol plus cisplatin versus gemzar plus cisplatin in treating advanced non-small cell lung cancer. Ai Zheng 2005;24:985-9. [PubMed]
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92-8. [Crossref] [PubMed]
- Gao YH, Guan WJ, Liu Q, et al. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016;21:269-79. [Crossref] [PubMed]
- Ohe Y, Niho S, Kakinuma R, et al. A phase II study of cisplatin and docetaxel administered as three consecutive weekly infusions for advanced non-small-cell lung cancer in elderly patients. Ann Oncol 2004;15:45-50. [Crossref] [PubMed]
- Stinchcombe TE, Choi J, Schell MJ, et al. Carboplatin-based chemotherapy in patients with advanced non-small cell lung cancer and a poor performance status. Lung Cancer 2006;51:237-43. [Crossref] [PubMed]
- Qin YY, Zhou CZ, Zhu Z, et al. Case report: dermatomyositis associated with lung cancer with heterogeneous morphology. J Thorac Dis 2017;9:E1110-7. [Crossref] [PubMed]
- Qin YY, Zhang DH, Lin XQ, et al. Clinical analysis of 36 cases of advanced non-small cell lung cancer (NSCLC) with performance status (PS) scores between 2 and 4. Zhonghua Zhong Liu Za Zhi 2017;39:855-61. [PubMed]


