Novel radiotherapy approaches for lung cancer: combining radiation therapy with targeted and immunotherapies
Role of radiation in early stage and locally advanced non-small cell lung cancer (NSCLC)
Definitive radiation therapy has been part of the standard of care for patients with locally advanced NSCLC for almost 5 decades. Combined modality therapy with chemoradiation became the preferred treatment of these patients based on multiple clinical trials showing improved survival (1,2). Conventionally fractionated radiation therapy remains the standard, and attempts at dose escalation have failed to show a benefit in this patient population (3). Newer technologies such as intensity modulated radiation (4), image guided radiation therapy, and proton therapy (5-7) are increasingly being utilized or studied to lower rates of toxicity with combined modality therapy.
Surgical resection has been the standard of care for patients with stage I NSCLC with 5 years survival rates of approximately 60-70% (8,9). While patients determined to be medically inoperable have been treated in the past with standard fractionated radiotherapy, newer technologies within radiation therapy have led to the standardization of high dose, ablative hypofractionated therapy termed stereotactic body radiation therapy (SBRT) or stereotactic ablative radiotherapy (SABR) (10). SBRT has allowed for improved dose conformity, improved local tumor control, and superior overall survival (OS) when compared to conventionally fractionated radiotherapy (11,12). Based on the improved outcomes with SBRT and the increased utilization of this technology, interest in its use for medically operable patients has emerged. A recently published pooled analysis of two randomized trials comparing surgery and SBRT for stage I NSCLC demonstrated that SBRT was highly effective and had a limited toxicity profile, and that there was equipoise between the two treatment options (13).
SBRT has also begun to be used more frequently in patients with oligometastatic disease, including lung, liver, and bone metastases. Recent data has shown excellent control rates with encouraging progression free survival (PFS) in patients with oligometastatic NSCLC (14,15). Conventionally fractionated radiotherapy, in combination with chemotherapy, can also be considered in patients with oligometastatic disease not amenable to treatment with SBRT and may improve survival in a select subset of patients with minimal extrathoracic disease (16).
Targeted therapy for advanced NSCLC
With the discovery of molecular pathways that correspond with tumor progression and growth, numerous potential targets have been identified and explored for potential therapeutics for advanced NSCLC (Table 1).
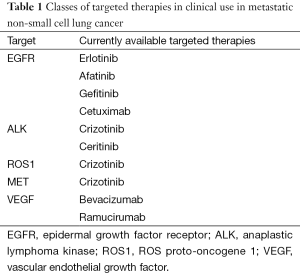
Full table
Epidermal growth factor receptor (EGFR) is an essential part of the oncogenic growth pathway and is expressed at higher levels in some lung cancers. EGFR as a molecular target has shown promising results in advanced lung cancer. Monoclonal antibodies, such as cetuximab and panitumumab, and tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and afatinib, are available. Initial trials evaluating patients treated with cytotoxic chemotherapy either in combination or followed by EGFR pathway inhibitors without prior molecular mutation analyses demonstrated mixed results, although trials have generally demonstrated at least a benefit to PFS (17-23). Further subset analysis of many of these trials showed clear correlation between the presence of EGFR driver mutations and clinical benefit of these agents. This has led to the standardization of the use of EGFR TKIs in the first line setting for patients with EGFR mutations (24-30).
Vascular endothelial growth factor (VEGF) plays an essential part in tumor angiogenesis and is often expressed at higher rates in NSCLC, thus creating another molecular pathway target for therapy. The most well studied VEGF inhibitor in NSCLC, bevacizumab, has shown increased PFS and OS in patients with non-squamous NSCLC when added to standard cytotoxic chemotherapy (31-33). Ongoing trials are evaluating bevacizumab with other platinum combinations (NCT00150657, NCT00753909), as well as with other targeted agents such as erlotinib and ramucirumab (NCT01532089, NCT00257608, NCT00553800).
One of the most promising recent areas of new drug development in treatment of NSCLC has been anaplastic lymphoma kinase (ALK) inhibitors. These are targeted agents directed at the novel fusion oncogene echinoderm microtubule associated protein like 4-anaplastic lymphoma kinase (EML4-ALK). The first available drug was crizotinib, an oral small-molecule inhibitor of ALK and c-Met tyrosine kinases. Crizotinib has shown favorable outcomes both in the second line setting, as well as in the primary treatment setting for patients that are positive for this rearrangement (34,35). Second generation TKI inhibitors of ALK include ceritinib and alectinib are undergoing investigation in national trials in ALK positive patients that have progressed, as well as the primary setting with pending results (NCT02292550, NCT02393625, NCT02075840, NCT02271139). ALK inhibitors have also demonstrated efficacy in patients with chromosomal rearrangements of the gene encoding ROS1 proto-oncogene receptor tyrosine kinase, which occurs in 1-2% of patients with NSCLC (36).
Immunotherapy for advanced NSCLC
Utilizing the immune system as an effective oncologic tool to fight cancer has been the subject of preclinical and clinical research for several decades (37). Immunotherapy agents allow the immune system to recognize a patient’s cancer cells as foreign, prompting an immune response resulting in tumor cell death and/or inhibition of tumor growth. Newer immunotherapy agents have been developed based on improved knowledge of the molecular process of the immune response, leading to a resurgence in investigative use of these agents for patients with NSCLC. Such checkpoint inhibitors include monoclonal antibodies to cytotoxic T-lymphocyte antigen 4 (CTLA-4) such as ipilimumab, as well as antibodies to programmed death receptor 1 (PD-1), such as nivolumab and pembrolizumab (Table 2).

Full table
CTLA-4 is responsible for regulation of early T cell activity. It becomes upregulated after antigen exposure and competes for binding with CD28, preventing the stimulatory signal needed for T cell activation. Thus, inhibition of this receptor allows T cell activation after tumor antigen presentation. PD-1 is also upregulated on T cells, but it is thought to play a role further down the immune response pathway within the tumor microenvironment. Binding of PD-1 to programmed death ligand 1 (PD-L1) leads to T cell inactivation, and antibodies to PD-1 allow activation to proceed at the site of direct anti-tumor immune response.
The majority of data for use of these newer immunotherapy agents in NSCLC have been studied in advanced, stage IV patients. Ipilimumab was developed as an IgG1 CTLA-4 monoclonal antibody and was originally investigated in metastatic melanoma. A phase II randomized trial combining ipilimumab with standard first line chemotherapy in patients with stage IIIB-IV NSCLC showed improvement of PFS with the addition of ipilimumab (38). Subset analysis showed that patients with squamous cell histology benefitted primarily from the addition of ipilimumab, prompting an ongoing phase III trial that is comparing standard first line chemotherapy with carboplatin and paclitaxel with or without the addition of ipilimumab in patients with advanced squamous cell NSCLC. Additional trials are evaluating its effectiveness in combination with other targeted or immunotherapy agents (39).
Anti PD-1 antibody agents have been more commonly studied in patients with progressive metastatic NSCLC and showed promising results with prolonged tumor responses (40). Based on the recently published data from the CheckMate 017 and 063 trials in 2014, nivolumab has now received Food and Drug Administration (FDA) approval for treatment of advanced squamous cell NSCLC. Checkmate 063 was a single arm phase II trial in patients that had progressed after at least two prior systemic treatments. Nivolumab achieved an encouraging 1 year survival rate of 41% in these heavily pretreated patients (41). The follow up phase III trial, CheckMate 017, randomized patients with metastatic squamous cell NSCLC who had progressed after doublet chemotherapy to nivolumab or and docetaxel. The trial was stopped early due to superior OS in the nivolumab arm with a median survival of 9.2 vs. 6 months in the docetaxel arm (P=0.00025). Nivolumab also showed a more favorable toxicity profile compared with docetaxel (42). Additional phase III trials are currently evaluating pembrolizumab monotherapy in both the first line and second line setting for advanced and metastatic NSCLC (NCT02220894, NCT02142738) (38).
Targeted therapy with radiation therapy for localized NSCLC
Many targeted therapies have been integrated into the treatment of localized NSCLC. While the data are much more limited than for the metastatic setting, targeted therapies have been used in combination with or concurrently with radiation therapy. The majority of this data are in conjunction with radiation therapy in the setting of locally advanced NSCLC classically treated with concurrent chemotherapy and radiation.
Preclinical data have shown biologic rationale for combining EGFR inhibitors and radiation therapy. Cetuximab has been combined with chemotherapy and radiation in treatment of locally advanced NSCLC in both phase II and phase III trials (3,43,44). In two sequential Radiation Therapy Oncology Group (RTOG) trials, cetuximab was combined with carboplatin/paclitaxel and radiation therapy for stage IIIA/IIIB lung cancer. While the median survival (22.7 months) and 24-month OS (49.3%) achieved in the phase II study (RTOG 0324) of cetuximab and concurrent chemoradiation were longer than any previously reported by the RTOG (43), the randomized phase III trial RTOG 0617 failed to show a benefit to the addition of cetuximab to chemoradiation in an unselected population (3). Among all patients, median OS in patients randomized to cetuximab was 25.0 vs. 24.0 months among those not receiving cetuximab (P=0.29). However, in a planned analysis of the association of EGFR expression and outcome, among patients with an EGFR H score of 200 or higher, cetuximab use was associated with improved OS (42.0 vs. 21.2 months, P=0.032) (3).
Gefitinib and erlotinib have also been integrated into both the concurrent chemoradiation setting, as well as a maintenance therapy after chemoradiation for locally advanced NSCLC (45-47). Again, phase III trials have failed to show a benefit to these agents in all subsets of patients, but they have shown improved outcomes in patients who had evidence of EGFR amplification or EGFR mutation, suggesting that in selected patients, these drugs may prolong PFS or OS in combination with chemotherapy and radiation therapy for non-metastatic patients. Newer studies are evaluating the use of these agents in patients with confirmed mutations (NCT01391260, NCT01822496, NCT02277457) (38).
Another area of clinical interest combining radiation and targeted therapy has been in the limited or oligometastatic setting. While the definition of oligometastatic has varied in the clinical literature, there has been increased use of local therapies for patients with limited sites of metastastic disease, especially as the ability to deliver effective local therapies with less morbidity has improved. Given the encouraging local control and limited toxicity profile of SBRT in both the lung and other organs commonly afflicted with metastasis from lung cancer, this remains an active area of research in treating patients with limited oligometastatic disease in combination with targeted agents. One recent published phase II trial showed encouraging results for PFS in advanced NSCLC patients with six or fewer sites of metastatic disease when they were treated with local SBRT to these sites in combination with second line erlotinib (7). Other active studies are similarly looking at this patient population in combination with other targeted as well as immunotherapeutic agents (NCT02450591, NCT0208672, NCT02444741).
As in the oligometastatic setting, the use of radiation therapy can be considered in the oligoprogression setting among patients being treated with TKIs for metastatic NSCLC. While patients with stage IV NSCLC and EGFR mutation or ALK rearrangement have achieved excellent PFS with targeted therapy, disease progression often occurs within a year of therapy initiation. While initial progression of EGFR- or ALK-directed therapy can be diffuse, many patients can have oligoprogression, or limited sites of progression, potentially due to acquired resistance from evolutionary selection on molecularly diverse tumors in which tumor clones in some sites of metastasis but not others develop resistance. Systemic options for such patients include increasing the dose of the targeted therapy they are progressing on, switching to another next-line targeted therapy, switching to cytotoxic chemotherapy, or adding chemotherapy to the targeted therapy (48). However, several groups have recently demonstrated that radiation therapy or other local therapies to sites of oligoprogression can also be considered and can achieve durable local control of the sites of progression and also allow for patients to be maintained on their existing TKI, thus saving alternative or next-line systemic therapy options for subsequent disease progression (49,50).
Anti-angiogenesis agents typically targeting VEGF have become standard treatment components of therapy for advanced NSCLC. Bevacizumab has been studied in combination with radiation therapy, but this combination has shown a high incidence of tracheoesophageal fistula formation when given concurrently, especially among patients with squamous cell carcinoma and centrally located tumors being irradiated (51).
Given the favorable results in advanced lung cancer, integration of ALK inhibitors into the setting of locally advanced NSCLC has already entered ongoing randomized phase II trials, including NRG/RTOG 1306/NCT01822496, which is evaluating erlotinib and crizotinib as induction therapy followed by standard chemoradiation in patients with confirmed EGFR mutation or EML4-ALK fusion rearrangement, respectively (39).
Immunotherapy with radiation therapy for NSCLC
Although there is limited data to date combining radiation therapy and immunotherapy, this combination has the ability to achieve a synergistic therapeutic effect (52,53). As ionizing radiation can increase the production and presentation of tumor antigens, it can serve to augment the antitumor immune responses achieved by checkpoint inhibitors (54). Radiation therapy can augment immunomodulation by bolstering cytotoxic T-lymphocyte activity (53) and reduce myeloid-derived suppressor cells (55), allowing for synergism with checkpoint inhibitors.
SBRT may be the radiotherapy modality most optimally combined with immunotherapy since it can achieve a more robust immune response than conventionally fractionated radiotherapy. SBRT has been shown to induce cellular expression of major histocompatibility complex (MHC) I, inflammatory mediators, costimulatory molecules, heat shock proteins, immunomodulatory cytokines, adhesion molecules, and death receptors, all of which can enhance antitumor immune responses of systemic therapy (56).
There have been a number of reports in which a distant tumor mass regresses following the administration of radiation therapy before or after treatment with immunotherapy, known as the abscopal effect (57-59). In addition to the abscopal effect, radiation therapy may also allow for immune activation that leads to a more complete or accelerated clearance of the irradiated tumor, or sterilization of microscopic metastasis that were not clinically apparent at the time of irradiation. Aside from case reports, a number of prospective clinical trials have been completed that have combined anti-CTLA-4 therapy and radiotherapy for melanoma (60) and prostate cancer (61) with promising results. A phase I/II study in metastatic castration resistant prostate cancer combining ipilimumab in combination with radiation therapy showed 50% of patients having a decline in prostate-specific antigen (PSA) with one complete response (60). A phase I trial combining ipilimumab and radiation in melanoma showed a response rate of 18% and PFS of 3.8 months prompting further investigation into this combination in the clinical setting (62). To date, no prospective study combining radiation therapy with anti-CTLA-4, anti-PD-1, or anti-PD-L1 therapy has been completed for lung cancer.
Future directions
Targeted therapy and immunotherapy have become pillars of lung cancer treatment. As we gain a greater understanding of the molecular basis of lung cancer, additional targeted agents will become part of standard practice to expand the role beyond the currently limited proportion of lung cancer patients with a known targetable mutation or translocation. Additionally, with increasing knowledge of acquired mutations, second- and third-line targeted agents will become standard options over salvage cytotoxic chemotherapy offering the promise of greater effectiveness and less toxicity. Cooperative group studies combining targeted agents and radiotherapy for non-metastatic patients are ongoing (NCT01822496).
Similarly, immunotherapies will become more entrenched as standard therapy for second-line NSCLC and will be investigated in the first line setting. Combination therapies will increasingly be the subject of investigation, including the inhibition of both CTLA-4 and PD-1, or the use of an immunotherapy agent with a targeted therapy or with a cytotoxic chemotherapy. Toxicities to such combinations, however, may prove prohibitive.
While there is much excitement around the phenomenon of a radiotherapy-induced anticancer immune response and combining radiation therapy with immunotherapy, numerous questions remain before this combination can be exported to routine clinical practice. Additional research is needed to determine if conventionally fractionated irradiation, multi-fraction SBRT, or single fraction SBRT is most effectively combined with immunotherapy, and how radiotherapy and immunotherapy should be sequenced. Like with combination systemic therapies, combining radiotherapy with such novel immunotherapies and systemic therapies may result in overlapping toxicities of radiation therapy and immunotherapy. In addition to the immune modulators and checkpoint inhibitors discussed in this manuscript, additional ways to provide tumor-associated antigen to the immune system that can be combined with radiotherapy are currently being investigated, including recombinant vaccines, tumor lysates, and synthetic peptides. While early results are promising, studies combining radiation therapy with immunotherapy warrant careful consideration of toxicity and safety.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [PubMed]
- Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692-9. [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [PubMed]
- Shirvani SM, Jiang J, Gomez DR, et al. Intensity modulated radiotherapy for stage III non-small cell lung cancer in the United States: predictors of use and association with toxicities. Lung Cancer 2013;82:252-9. [PubMed]
- Wink KC, Roelofs E, Solberg T, et al. Particle therapy for non-small cell lung tumors: where do we stand? A systematic review of the literature. Front Oncol 2014;4:292. [PubMed]
- Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer 2011;117:4707-13. [PubMed]
- Simone CB 2nd, Rengan R. The use of proton therapy in the treatment of lung cancers. Cancer J 2014;20:427-32. [PubMed]
- Naruke T, Goya T, Tsuchiya R, et al. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg 1988;96:440-7. [PubMed]
- Nesbitt JC, Putnam JB Jr, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg 1995;60:466-72. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Heinzerling JH, Kavanagh B, Timmerman RD. Stereotactic ablative radiation therapy for primary lung tumors. Cancer J 2011;17:28-32. [PubMed]
- Simone CB 2nd, Wildt B, Haas AR, et al. Stereotactic body radiation therapy for lung cancer. Chest 2013;143:1784-90. [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [PubMed]
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824-30. [PubMed]
- Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: a systematic review. J Thorac Oncol 2010;5:1091-9. [PubMed]
- Xanthopoulos EP, Handorf E, Simone CB 2nd, et al. Definitive dose thoracic radiation therapy in oligometastatic non-small cell lung cancer: A hypothesis-generating study. Pract Radiat Oncol 2015;5:e355-63. [PubMed]
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31. [PubMed]
- Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010;28:911-7. [PubMed]
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010;11:521-9. [PubMed]
- Brugger W, Triller N, Blasinska-Morawiec M, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J Clin Oncol 2011;29:4113-20. [PubMed]
- Takeda K, Hida T, Sato T, et al. Randomized phase III trial of platinum-doublet chemotherapy followed by gefitinib compared with continued platinum-doublet chemotherapy in Japanese patients with advanced non-small-cell lung cancer: results of a west Japan thoracic oncology group trial (WJTOG0203). J Clin Oncol 2010;28:753-60. [PubMed]
- Zhang L, Ma S, Song X, et al. Gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804): a multicentre, double-blind randomised phase 3 trial. Lancet Oncol 2012;13:466-75. [PubMed]
- Zhao H, Fan Y, Ma S, et al. Final overall survival results from a phase III, randomized, placebo-controlled, parallel-group study of gefitinib versus placebo as maintenance therapy in patients with locally advanced or metastatic non-small-cell lung cancer (INFORM; C-TONG 0804). J Thorac Oncol 2015;10:655-64. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866-74. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 2010;21:1804-9. [PubMed]
- Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013;24:20-30. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [PubMed]
- Smith RT. Tumor-specific immune mechanisms. N Engl J Med 1968;278:1326-31 concl.
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- ClinicalTrials.gov. A service of the U.S. National Institutes of Health. [Accessed September 29, 2015]. Available online: https://clinicaltrials.gov/
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Ramalingam SS, Mazières J, Planchard D, et al. Phase II Study of Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in Patients with Advanced, Refractory Squamous Non-Small Cell Lung Cancer: Metastatic Non-small Cell Lung Cancer. Int J Radiat Oncol 2014;90:1266-7.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [PubMed]
- Blumenschein GR Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 2011;29:2312-8. [PubMed]
- Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 2011;29:3120-5. [PubMed]
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005;23:5892-9. [PubMed]
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol 2007;25:1545-52. [PubMed]
- Ready N, Jänne PA, Bogart J, et al. Chemoradiotherapy and gefitinib in stage III non-small cell lung cancer with epidermal growth factor receptor and KRAS mutation analysis: cancer and leukemia group B (CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol 2010;5:1382-90. [PubMed]
- Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol 2014;11:473-81. [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [PubMed]
- Yu HA, Sima CS, Huang J, et al. Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:346-51. [PubMed]
- Spigel DR, Hainsworth JD, Yardley DA, et al. Tracheoesophageal fistula formation in patients with lung cancer treated with chemoradiation and bevacizumab. J Clin Oncol 2010;28:43-8. [PubMed]
- Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res 2015;4:177-90. [PubMed]
- Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res 2014;2:831-8. [PubMed]
- Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006;203:1259-71. [PubMed]
- Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687-95. [PubMed]
- Finkelstein SE, Timmerman R, McBride WH, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol 2011;2011:439752.
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [PubMed]
- Stamell EF, Wolchok JD, Gnjatic S, et al. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013;85:293-5. [PubMed]
- Hiniker SM, Chen DS, Reddy S, et al. A systemic complete response of metastatic melanoma to local radiation and immunotherapy. Transl Oncol 2012;5:404-7. [PubMed]
- Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373-7. [PubMed]
- Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013;24:1813-21. [PubMed]
- Mayor S. Radiation in combination with immune-checkpoint inhibitors. Lancet Oncol 2015;16:e162. [PubMed]


