
The best regimens for chemo-naïve incurable non-squamous non-small cell lung cancer with a programmed death-ligand 1, tumor proportion score 1–49%: a network meta-analysis
Introduction
Non-small cell lung cancer (NSCLC) is one of the most common cancers and the leading cause of cancer-related death worldwide (1). Platinum doublet and triplet chemotherapies have been the standard of care for patients with inoperable NSCLC without actionable driver mutations or translocations. The development of immune checkpoint inhibitors (ICIs) has offered enhanced therapeutic options for a variety of malignancies (2). ICIs are effective for multiple types of cancers because they treat malignant tumors by blocking checkpoint proteins but not directly attacking tumor cells. For example, programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) inhibitors prevent tumor cells from inactivating the immune system (3). Currently, the first-line therapeutic option for chemo-naïve patients with stage IV PD-L1-high [tumor proportion score (TPS) 50% or higher] NSCLC without driver alterations is single-agent pembrolizumab (Pemb) (4-7). Several regimens combining ICIs, bevacizumab (Bev), and cytotoxic drugs are recommended even for patients with low or no PD-L1 expression (4-7), since they greatly improve the objective response rate, progression-free survival (PFS), and overall survival (OS) of patients without substantially increasing the risk of adverse events compared to reference platinum regimens without ICIs. Although kinase inhibitors targeting epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), and B-raf proto-oncogene (BRAF) are highly effective for patients with specific actionable mutations or translocations, such kinase inhibitors are not recommended for NSCLC patients without these mutations or translocations (8).
Few randomized clinical trials (RCTs) have directly compared ICI regimens because such a trial requires a large number of participants to reveal small differences in outcomes. However, understanding differences in efficacy and safety among ICI regimens is vital because they are crucial for treatment selection. A network meta-analysis is the best analytical technique for enabling indirect comparison among multiple regimens. Our previous network meta-analysis published in 2017 evaluated only non-ICI, non-kinase inhibitor regimens for chemo-naïve incurable NSCLC (9). The aim of the current study is to update the previous network meta-analysis, focusing on ICI regimens and low PD-L1 expression (TPS 1–49%) in non-squamous NSCLC cases without driver alterations. We present the following article in accordance with PRISMA NMA reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-419) (10).
Methods
Protocol registration and overview
The protocol of this study followed the PRISMA extension statement for network meta-analysis (10), and the study was registered at the University Hospital Medical Information Network Center, Japan (11). There are amendments to information in the protocol (Appendix 1). Patient informed consent and institutional review board approval were not required for a systematic review.
Study search
The MEDLINE, Embase, Web of Science Core Collection, and Cochrane Central Register of Controlled Trials databases were searched to identify eligible articles on October 15, 2020. The formula used for MEDLINE is shown in Appendix 2. Conflicts during the study selection were resolved by discussion between the two reviewers (NF and SK) or inquiry to a third reviewer (NH).
Inclusion criteria: publication type and trial design
Individually randomized controlled trials of incurable NSCLC written in English were collected. Studies focusing on patients with driver mutations or translocations were excluded. A conference abstract was allowed. Trials including random assignment to maintenance therapy, second-line treatment, or later-line treatments were excluded.
Inclusion criteria: treatment
Eligible treatments included first-line chemotherapy, including cytotoxic agents, molecular targeted therapies, and ICIs. Trials adding angiogenesis inhibitor to the platinum regimen were included. ICIs could be used alone or in combination with platinum regimens.
While comparability between cisplatin (CDDP) and carboplatin (CBDCA) in the treatment of NSCLC is controversial (12,13), recent trials have allowed either CDDP or CBDCA based on the physician’s choice (14,15). Similarly, one of the important RCTs regarding Pemb, Keynote-047, included a “CBDCA and either paclitaxel (Ptx) or nanoparticle albumin-bound Ptx (nabPtx)” arm. Therefore, we decided to allow selective administration of CDDP/CBDCA and Ptx/nabPtx for our analysis. Nedaplatin was distinguished from CDDP and CBDCA.
Some recent trials that evaluated adding ICIs to platinum regimens allowed physicians to choose from several platinum regimens; however, a regimen-based network meta-analysis cannot incorporate such trials. Therefore, two models were constructed for analysis. One model, which was termed the “main model”, did not distinguish each platinum + third generation cytotoxic agent regimen (platinum regimen). The other model, which was termed the “separate model”, recognized each platinum regimen individually.
Kinase inhibitors were beyond the scope of the study because a regimen with these medications was not standard for patients without driver alterations (4-7).
RCTs examining perioperative chemotherapy and combined chemoradiotherapy were excluded from this study.
Inclusion criteria: patients
Patients with advanced, locally advanced, or recurrent non-squamous NSCLC were included. We accepted the disease stage that was mentioned in an article regardless of the historical revisions of the tumor-node-metastasis classification. Patients were not excluded if they had a medical history of radiotherapy or surgery. However, one study focusing on patients with poor performance status and the elderly was excluded. An RCT of large cell neuroendocrine carcinoma was excluded.
Patients whose PD-L1 protein expression as determined by the TPS was 50% or higher were excluded because current guidelines recommend different treatment options for those with a TPS <50% and those with a TPS ≥50%. Patients with a TPS <1% were also excluded because the number of patients with a TPS <1% influenced the results. If a subset of the study population fit our criteria, the subset data were analyzed. For example, when a study separately provided data of three populations with TPS scores of 0%, 1–49%, and ≥50%, we collected the data of the population with a TPS 1–49%.
If a study focused on patients with squamous NSCLC, driver alterations, or a TPS ≥50%, the study was excluded. However, a study without criteria regarding the pathological subclassification of NSCLC, driver gene mutations, and TPS was acceptable; otherwise, most NSCLC studies would have been excluded.
Quality assessment
The Cochrane Risk of Bias tool was used for the quality assessment. This assessment tool included selection, performance, detection, attrition, reporting, and other bias (16).
Outcomes
The primary outcome of this analysis was OS, evaluated using hazard ratio (HRos). The secondary endpoints were the HR for PFS (HRpfs), the odds ratio of adverse events based on the Common Terminology Criteria for Adverse Events grade III or higher (ORae) (17), and the OR of treatment-related death. Disease progression was assessed in compliance with the Response Evaluation Criteria in Solid Tumors guidelines published in 2000 or its 2009 revision (18). Imaging evaluation performed by blinded independent central reviewing was preferred, if available. The first adverse event of grade III or higher was counted even if a patient experienced two or more adverse events.
Data extraction
Characteristics of eligible studies, including the first author name, publication year, sample size, and trial outcome, such as HRos and its 95% confidence interval (CI), were extracted by two reviewers (NF and SK). Consensus was reached by discussion between the two reviewers and arbitration from a third reviewer (NH). Survival updates were included if available. Parmar’s method was accepted for the survival data (19). If available, data from intention-to-treat analyses were selected. The treatment arm was named based on the drug combination, regardless of the dose, route, or schedule. We obtained data from a subgroup by subtraction using a fixed-model meta-analysis formula. For example, subtracting data of the “PD-L1 1–49%” subgroup from data of the “PD-L1 <50%” subgroup yielded the data of the “PD-L1 <1%” subgroup. The data on adverse events and treatment-related death could be for patients with any PD-L1 TPS or pathology because these stratified data were rarely described.
Statistical analyses
The frequentist weighted least squares approach random-model network meta-analysis was applied in our study (20). The OR of a binary outcome was continuously corrected with 0.5, if necessary. The OR and HR were log-converted. In the separate model, platinum + pemetrexed (Pemt) was used as a common reference comparator because this regimen was selected for non-squamous carcinoma in recent trials (14,15). Data were analyzed using R software (Command: netmeta, Package: netmeta) (21). The significance threshold was set at P<0.05.
Results
Study search
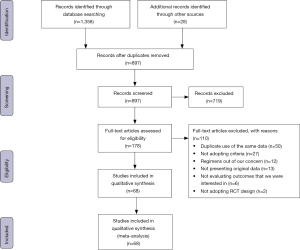
We identified 1386 articles by electronic and manual searches. Of the 897 articles that met the preliminary criteria, 489, 719, and 110 were excluded through removal of duplicated studies, title/abstract screening, and full article review, respectively. We identified 68 eligible studies (Figure 1, Appendix 3).
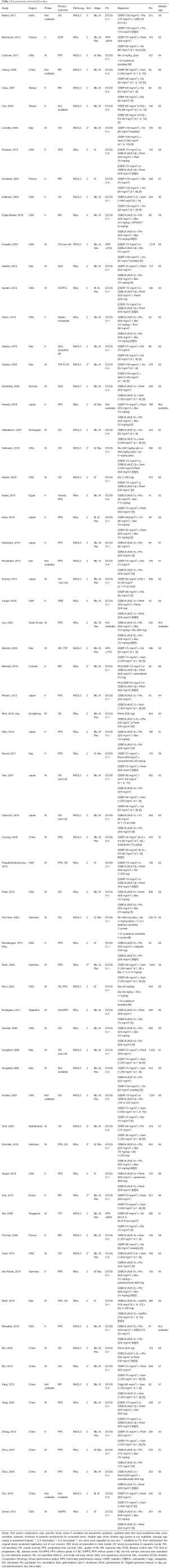
Characteristics of the included studies
The main model had 26 studies and 53 arms, of which 16 included ICIs. The separate model had 63 studies and 130 arms, of which 9 included ICIs. The median and average age of patients ranged from 51 years to 67 years, with 50 studies having a median/average age in the 60s. The total number of patients was 22,619, and the number of randomized patients in each study ranged from 41 to 1,252, with a median of 248 (Table 1, Appendix 4).
Full table
According to the Cochrane Risk of Bias evaluation, all but one of the studies had at least one domain with a high risk of bias (Table S1).
Efficacy analysis
Data for HRos were obtained in 26 studies with 7,142 patients (Table 1). In the main model, the HRos of 23 pairwise comparisons ranged from 0.55 to 1.64, with a median of 0.94. Q statistics and a test for heterogeneity did not reveal inconsistency at any level (whole network level I2=0%, total; P=0.348, within designs; P=0.348) (Figure 2, Figure S1). Eligible treatments were clustered into the same node. The platinum regimen + Pemb (HRos =0.55, 95% CI: 0.34–0.89, P=0.015) showed the best OS, followed by the platinum regimen + nivolumab (Niv) + ipilimumab (Ipi) (HR = 0.61, 95% CI: 0.44–0.84, P=0.003) (Figure 3A). The HRos of these regimens were significant against the platinum regimen. The HRos of the other regimens were not significantly different from that of the platinum regimen. The platinum regimen + atezolizumab (Atz) (HR =0.70, 95% CI: 0.45–1.08, P=0.110) did not show superiority to the platinum regimen in terms of OS. We conducted a subgroup analysis excepting conference abstracts, which did not conflict with the main analysis with the conference abstract (Figure S2). In the separate model, the platinum regimen + Pemt + Pemb (HR =0.55, 95% CI: 0.34–0.89, P=0.014) showed the best OS. This regimen was significantly different in the separate model (Figure S3).
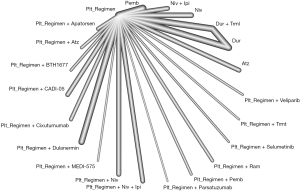
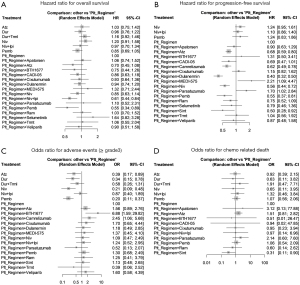
The HRpfs of the platinum regimen + Pemb were significantly decreased compared to the platinum regimen alone (HRpfs =0.55, 95% CI: 0.37–0.81, P=0.003) (Figure 3B). The lowest HRpfs was observed in the platinum regimen + dulanermin (HRpfs =0.40, 95% CI: 0.32–0.50, P<0.001), followed by the platinum regimen + sintilimab (Sint), the platinum regimen + Pemb, the platinum regimen + Niv, the platinum regimen + camrelizumab, and the platinum regimen + Atz.
Safety analysis
The lowest risk of grade III adverse events was observed in the Pemb arm (OR =0.20, 95% CI: 0.11–0.37, P<0.001) against the platinum regimen, followed by Niv, durvalumab, and Atz (Figure 3C,3D). Regarding chemotherapy-related death, there was no significant difference in regimens except the platinum regimen + Sint (OR =0.31, 95% CI: 0.11–0.90, P=0.029).
Discussion
We carried out the first network meta-analysis to compare regimens including cytotoxic agents, molecular targeted therapies, and ICIs for chemo-naïve incurable NSCLC with low PD-L1 expression. The network method was able to concurrently compare a variety of chemotherapy regimens. Moreover, the sufficient statistical power supported by the substantial studies ensured the validity of the results.
The immune response activated by PD-L1 inhibition is enhanced by cytotoxic chemotherapy, which reduces regulatory T-cell activity (22). Combination therapy is expected to improve the anticancer activity. Among the 22 regimens, the HRos of the platinum regimen + Pemb and the platinum regimen + Niv + Ipi were 0.55 (95% CI: 0.34–0.89) and 0.61 (95% CI: 0.44–0.84), respectively, with the platinum regimen alone as the reference. These regimens in this order showed the best performance in terms of OS (Figure 3A). The platinum regimen + Pemb also showed a high rank in terms of improving PFS (Figure 3B). Combination therapy is said to provide an early disease control relative to ICI monotherapy (15), preventing early disease progression. Moreover, less than half of patients with advanced NSCLC receive second-line therapy (23). Patients treated with monotherapy may miss the opportunity to receive other regimens.
The adverse events of the platinum regimen + Pemb (ORae =1.30, 95% CI: 0.68–2.49) and the platinum regimen + Niv + Ipi (ORae =1.24, 95% CI: 0.52–2.95) were not significantly greater than those of the platinum regimen alone (Figure 3C). We recommend the platinum regimen + Pemb or the platinum regimen + Niv + Ipi when the PD-L1 TPS is 1–49%. By contrast, there was no significant difference between the HRos of ICI monotherapy and the platinum regimen; therefore, ICI monotherapy is not recommended. However, ICI monotherapy tends to have a low risk of adverse events. The ORae with Pemb monotherapy was the lowest in this study (ORae =0.20, 95% CI: 0.11–0.37) (Figure 3C). These regimens can be considered if the patients are elderly and have a low PS.
The platinum regimen + dulanermin did not have a significant effect on OS compared to the platinum regimen, but this regimen had the lowest HRpfs. Dulanermin is recombinant TRAIL/Apo2L, a novel molecular target, which can induce apoptosis in tumor cells. However, dulanermin is administered intravenously on days 1 to 14 in a cycle until disease progression.
When a patient fulfills the criteria of this study, the Japanese Lung Cancer Society Guideline (4) recommends adding Atz to the platinum regimen in accordance with the guidelines of the American Society of Clinical Oncology, National Comprehensive Cancer Network, and European Society for Medical Oncology. Our study did not show a significant effect of the Atz regimen on OS (HR =0.70, 95% CI: 0.45–1.08) (Figure 3A). Impower 150 (24), which compared platinum + Ptx + Bev + Atz and platinum + Ptx + Bev, indicated that adding Atz was effective in terms of PFS (HRpfs =0.68, 95% CI: 0.56–0.82, P<0.001), but the HRos in patients with low PD-L1 expression (1–49%) was not shown.
There were some limitations to our study. First, most of the evaluated original trials had a high risk of bias, as judged by the Cochrane tool. Unfortunately, in practical terms, it is difficult to conduct a double-blinded trial without sponsorship, and we believe that these factors do not largely reduce the credibility. Second, because the main model regarded each platinum regimen as identical, the results may not be accurate. However, we believe that the two models have similar consequences. Thirdly, different PD-L1 assays were usually selected for pathological specimen based on the ICI drug selection. Furthermore, there is no universally accepted judgement for PD-L1 positivity. As a result, even though we tried to find out original studies with cutoff value of PD-L1 1% and 50%, such cutoff values might be slightly different among studies (25). In conclusion, we conducted a systematic review and network meta-analysis examining ICIs in patients with non-squamous NSCLC with low PD-L1 expression. Based on 20,257 NSCLC patients constituting 59 RCTs, the platinum regimen + Pemb and the platinum regimen + Niv + Ipi seem to be reasonable first-line regimens for non-squamous NSCLC with a PD-L1 TPS 1–49%.
Acknowledgments
We would like to thank Editage (
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA NMA reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-419
Peer Review File: Available at https://dx.doi.org/10.21037/tlcr-21-419
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-419). Dr. NH has received personal fee from Taiho Pharmaceutical and research grant from Taiho Pharmaceutical outside of the work. Dr. KW has received personal fee from AstraZeneca, Ono Pharmaceutical and Boehringer Ingelheim outside of the work. Dr. YH has received personal fee from AstraZeneca and Boehringer Ingelheim outside of the work. Dr. NK has received personal fee from Chugai Pharmaceutical, AstraZeneca, Boehringer Ingelheim, Sanofi, Ono Pharmaceutical, MSD, Bristol Myers Squibb, Eli Lilly, Kyowa Kirin and research grant from Chugai Pharmaceutical, Boehringer Ingelheim, MSD, Eli Lilly, Kyowa Kirin, Daiichi Sankyo, Pfizer outside of the work. Dr. TK has received personal fee from Chugai Pharmaceutical, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Taiho Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo, Sanofi, Pfizer and research grant from MSD, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, Chugai Pharmaceutical, Daiichi Sankyo, Pfizer, Shionogi outside of the work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561-84. [Crossref] [PubMed]
- Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98-106. [Crossref] [PubMed]
- Akamatsu H, Ninomiya K, Kenmotsu H, et al. The Japanese Lung Cancer Society Guideline for non-small cell lung cancer, stage IV. Int J Clin Oncol 2019;24:731-70. [Crossref] [PubMed]
- Hanna NH, Schneider BJ, Temin S, et al. Therapy for stage IV Non-Small-Cell lung cancer without driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol 2020;38:1608-32. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: non-small cell lung cancer. 2020. Available online: https://www.nccn.org (accessed 14 April 2021).
- European Society for Medical Oncology. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up: updated version published 15. 2020. Available online: https://www.esmo.org (accessed 13 April 2021).
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637-58. [Crossref] [PubMed]
- Horita N, Nagashima A, Nakashima K, et al. The best platinum regimens for chemo-naive incurable non-small cell lung cancer: network meta-analysis. Sci Rep 2017;7:13185. [Crossref] [PubMed]
- Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. [Crossref] [PubMed]
- University Hospital Medical Information Network (UMIN) Center. Available online: https://www.umin.ac.jp/ctr/ctr_regist.htm (accessed 15 October 2020).
- Hotta K, Matsuo K, Ueoka H, et al. Meta-analysis of randomized clinical trials comparing Cisplatin to Carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol 2004;22:3852-9. [Crossref] [PubMed]
- Griesinger F, Korol EE, Kayaniyil S, et al. Efficacy and safety of first-line carboplatin-versus cisplatin-based chemotherapy for non-small cell lung cancer: a meta-analysis. Lung Cancer 2019;135:196-204. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Atkinson TM, Ryan SJ, Bennett AV, et al. The association between clinician-based common terminology criteria for adverse events (CTCAE) and patient-reported outcomes (PRO): a systematic review. Support Care Cancer 2016;24:3669-76. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998;17:2815-34. [Crossref] [PubMed]
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods 2012;3:80-97. [Crossref] [PubMed]
- Rucker G. Package “netmeta”: network meta-analysis using frequentist methods. 2016. Available online: https://cran.r-project.org/web/packages/netmeta/netmeta.pdf#search= (accessed 14 April 2021).
- Zitvogel L, Galluzzi L, Smyth MJ, et al. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013;39:74-88. [Crossref] [PubMed]
- Davies J, Patel M, Gridelli C, et al. Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: a systematic review of recently published studies. PLoS One 2017;12:e0175679 [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Paver EC, Cooper WA, Colebatch AJ, et al. Programmed death ligand-1 (PD-L1) as a predictive marker for immunotherapy in solid tumours: a guide to immunohistochemistry implementation and interpretation. Pathology 2021;53:141-56. [Crossref] [PubMed]


