
The long-term oncologic outcomes of robot-assisted bronchial single sleeve lobectomy for 104 consecutive patients with centrally located non-small cell lung cancer
Introduction
Bronchial sleeve lobectomy has been widely considered as the preferred alternative to pneumonectomy for centrally located non-small cell lung cancer (NSCLC) (1,2). Several meta-analyses have shown that sleeve lobectomy can reduce postoperative complications and improve long-term survival and quality of life, without any increase in recurrence rates (1-3). With the development of robotic surgery systems and technology, such as three-dimensional magnified vision, EndoWrist instruments and tremor filtration technology allow surgeons to operate more flexibly and safely, especially during complex operations such as sutures in sleeve resections (4-9). In our institution, Jiao and colleagues reported a series of 67 patients who underwent robotic single sleeve lobectomy (9). The half-continuous suturing technique recommended in the report could effectively simplify and replace the traditional complex intermittent suture method in sleeve lobectomy. Furthermore, the operation could be completed safely and efficiently (9,10). Recent studies have confirmed that robot-assisted bronchial sleeve resection was clinically feasible in terms of postoperative clinical outcomes and short-term survival (4-8,11). Several studies have reported the mid-term survival of robot-assisted sleeve lobectomy, including 2- and 3-year survival data (9-11). In the cohort of patients undergoing bronchial sleeve resection including robot-assisted sleeve lobectomy, Gu et al. (11) indicated that tumor size, postoperative radiotherapy and intensive care unit (ICU) stay were predictors of survival, while Qiu et al. (10) reported that positive bronchial margin, pathologic T4 stage and N2 stage were risk factors of poor prognosis. However, until now, there have been no cohort studies examining the long-term survival and its prognostic factors of robot-assisted sleeve lobectomy, which means that there is a lack of feasibility evaluation of the technique in terms of long-term oncologic outcomes.
Therefore, in the present study, we assessed the long-term oncologic outcomes in a series of 104 patients to further reinforce the oncological feasibility of sleeve lobectomy via robotic surgical systems. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-298/rc).
Methods
Patient cohort
Consecutive patients with centrally located NSCLC who underwent robot-assisted bronchial single sleeve lobectomy from October 2014 to May 2021 were retrospectively reviewed. Bronchial single sleeve lobectomy only refers to the resection and end-to-end anastomosis reconstruction of the bronchus, without the resection of the pulmonary vessels or carina. Exclusion criteria were patients with sleeve pneumonectomy, sleeve segmentectomy, pulmonary angioplasty, carina resection, or patients who were lost to follow-up. A total of 104 patients who underwent robot-assisted bronchial single sleeve lobectomy were enrolled in this retrospective study. The baseline demographics, operation information, pathologic features, and clinical outcomes of all patients were collated from electronic medical records. Preoperative comorbidities were assessed according to the Charlson Comorbidity Index (CCI) (12). All tumors were classified according to the eighth edition of TNM classification for lung cancer (13). Survival outcomes of patients were collected via outpatient clinic records and telephone follow-up. The end time of follow-up was August, 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the medical ethics committee of the Affiliated Hospital of Qingdao University (No. QYFYWZLL 26574). Individual consent for this retrospective analysis was waived.
Patient management
All patients received routine preoperative evaluations, including chest computed tomography (CT) scan or contrast-enhanced CT, flexible bronchoscopy, abdominal CT scan, brain CT scan or magnetic resonance imaging (MRI), bone scan, cervical lymph nodes (LNs) ultrasonography, pulmonary function test, and echocardiography. Positron emission tomography CT scan (PET-CT) or endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) was performed if necessary. Postoperative treatment and nursing strategies were standardized for all patients. Bronchial anastomosis was confirmed by fibrotic bronchoscopy before patients were discharged from the hospital.
Chest CT scans were performed one month after surgery, and at intervals of 3 to 6 months thereafter. Brain or bone MRI scans were performed if the patient showed any signs or symptoms of recurrence. PET-CT scans or biopsies were recommended to confirm any suspected recurrence or metastasis. The overall survival (OS) was defined as the time from surgery to death or the last follow-up. Disease-free survival (DFS) was defined as the interval from surgery to recurrence, metastasis, last follow-up, or death. Recurrence status was categorized as locoregional or distant.
Surgical technique
Robot-assisted bronchial sleeve lobectomy was performed using the da Vinci Surgical System (Intuitive Surgical, Inc., Mountain View, CA, USA). The relevant technologies of robotic surgery we used have been published in our former studies (9,10,14,15). Systematic dissection of LNs was usually completed, including at least three hilar stations and three mediastinal stations of LNs. Bronchial proximal and distal stumps were confirmed to be radical resections (R0) by intraoperative frozen sections. Bronchial extended resection or alternative pneumonectomy was performed when the frozen sections showed the microscopic residual disease on the margin (R1), until the negative bronchial margin was confirmed. After completion of the bronchial anastomosis, a sealing test was performed under water to confirm no air leakage at the bronchial anastomosis. Before closing the thoracic cavity, one chest tube was placed to the apical thorax for drainage.
Statistical analysis
Continuous variables are summarized as mean ± standard deviation (SD). Categorical variables are summarized as number and proportion. Survival was estimated using the Kaplan-Meier method, and the significant difference of nonparametric groups was assessed using the log rank test. Cox proportional hazards regression models were performed to determine the prognostic value of risk factors identified in the univariate and multivariate analyses. Risk factors with P<0.1 for DFS and OS in univariable analyses were subsumed into the multivariable Cox analysis. A P value <0.05 was considered statistically significant, and all P values were two-sided. All statistical analyses were performed using the IBM SPSS Statistics version 25.0 software (IBM Corporation, NY).
Results
In the present study, a total of 104 consecutive patients with pathological stage I–III NSCLC who received robot-assisted bronchial single sleeve lobectomy were identified. The characteristics of all patients is shown in Table 1. In the total cohort, there were 89 (85.6%) males and 15 (14.4%) females, with an average age of 61.6 years. The majority of the reviewed patients (81/104, 77.9%) had a history of smoking. The average CCI was 2.1 for all patients. In terms of pulmonary function, the mean of the forced expiratory volume in one second (FEV1) was 2.6±0.6 L. The average percentage of predicted FEV1 (FEV1%pred) was 92.2%±16.7% and the percentage of predicted diffusing capacity of the lung for carbon monoxide (DLCO%pred) was 94.0%±17.8%.
Table 1
| Characteristic | Total (N=104) |
|---|---|
| Gender, n (%) | |
| Male | 89 (85.6) |
| Female | 15 (14.4) |
| Age (years), mean ± SD | 61.6±9.0 |
| BMI (kg/m2), mean ± SD | 24.4±3.3 |
| Smoking history, n (%) | |
| Never | 23 (22.1) |
| Current/former | 81 (77.9) |
| CCI, mean ± SD | 2.1±1.1 |
| Pulmonary function, mean ± SD | |
| FEV1 (L) | 2.6±0.6 |
| FEV1%pred | 92.2±16.7 |
| DLCO%pred | 94.0±17.8 |
| Neoadjuvant treatment, n (%) | 21 (20.2) |
SD, standard deviation; BMI, body mass index; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity of lung for carbon monoxide; %pred, percentage predicted.
Of the 104 patients, 74 (71.2%) were diagnosed with squamous cell carcinoma (SCC) as described in Table 2. A total of 41 (39.4%) patients presented with LN metastasis (N1, 16.3%; N2, 23.1%). All patients had M0 stage disease. In terms of pathological features, 13 (12.5%) patients presented with visceral pleura invasion (VPI), 17 (16.3%) patients had lymphovascular invasion (LVI), and 18 (17.3%) patients had necrosis of the tumor. The number of LNs removed was 23.8±8.9. The average number of stations for LNs removed was 6.6±1.1. Most patients had tumors in the upper lobe (67/104, 64.4%). A total of 8 (7.7%) patients received robot-assisted sleeve bilobectomy. All patients achieved R0 resection. After the operation, 49 (47.1%) patients received adjuvant therapy.
Table 2
| Characteristic | Total (N=104) |
|---|---|
| Histological status, n (%) | |
| Squamous cell carcinoma | 74 (71.2) |
| Adenocarcinoma | 12 (11.5) |
| Others | 18 (17.3) |
| Pathological T stage, n (%) | |
| T1 | 11 (10.6) |
| T2 | 75 (72.1) |
| T3 | 14 (13.5) |
| T4 | 4 (3.8) |
| Pathological N stage, n (%) | |
| N0 | 63 (60.6) |
| N1 | 17 (16.3) |
| N2 | 24 (23.1) |
| Pathological stage, n (%) | |
| I | 47 (45.2) |
| II | 28 (26.9) |
| III | 29 (27.9) |
| VPI, n (%) | |
| Absent | 91 (87.5) |
| Present | 13 (12.5) |
| LVI, n (%) | |
| Absent | 87 (83.7) |
| Present | 17 (16.3) |
| Necrosis, n (%) | |
| Absent | 86 (82.7) |
| Present | 18 (17.3) |
| Number of LNs removed, mean ± SD | 23.8±8.9 |
| Stations of LNs removed, mean ± SD | 6.6±1.1 |
| Sleeve lobectomy site, n (%) | |
| Upper lobe | 67 (64.4) |
| Middle lobe | 3 (2.9) |
| Lower lobe | 26 (25.0) |
| Right double-lobe | 8 (7.7) |
| Adjuvant therapy, n (%) | 49 (47.1) |
| Recurrence status, n (%) | |
| No recurrence | 78 (75.0) |
| Locoregional | 10 (9.6) |
| Distant | 16 (15.4) |
VPI, visceral pleura invasion; LVI, lymphovascular invasion; SD, standard deviation; LN, lymph node.
During the follow-up, recurrence occurred in 26 (25.0%) patients in total, including locoregional recurrence in 10 (9.6%) patients and distant recurrence in 16 (15.4%) patients. No endobronchial or perianastomotic recurrence occurred. The median follow-up time was 45.0 months. Kaplan-Meier curves of OS and DFS are shown in Figure 1.
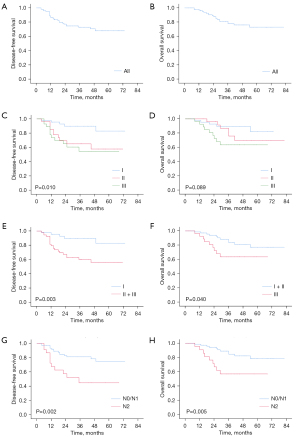
Kaplan-Meier analysis demonstrated that the 5-year DFS rate was 67.9% and the 5-year OS rate was 73.0% in the entire retrospective cohort. Stratified survival data were calculated according to pathological stages. For stage I patients, the 5-year DFS and OS rates were 82.9% and 82.2%, respectively. For stage II patients, the 5-year DFS and OS rates were 57.8% and 69.7%, respectively. The 5-year DFS and OS rates for patients with stage III disease were 54.5% and 63.7%, respectively. The DFS rate of patients with stage II and stage III disease was significantly worse than that of stage I patients (P=0.003). Meanwhile, the OS rate of stage III patients was significantly poorer than that of patients with stage I and stage II disease (P=0.040). There was no significant difference in survival between N0 stage and N1 stage patients as shown in Table 3 (DFS, P=0.898; OS, P=0.764). Therefore, the N0 and N1 stages were analyzed in one group. For patients with N0 or N1 diseases, the 5-year DFS and OS rates were 75.1% and 78.5%, respectively. Meanwhile, the 5-year DFS and OS rates were 44.9% and 57.0% for patients with N2 disease. The survival rate of N2 patients was significantly worse than that of N0/N1 patients (DFS, P=0.002; OS, P=0.005).
Table 3
| Characteristics | DFS | OS | |||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Gender (female) | 0.580 (0.366–0.918) | 0.020 | 2.338 (0.778–7.022) | 0.130 | |
| Age | 0.973 (0.933–1.014) | 0.193 | 0.998 (0.950–1.049) | 0.948 | |
| BMI | 1.028 (0.909–1.163) | 0.660 | 0.934 (0.804–1.085) | 0.375 | |
| Smoking history (current/former) | 0.501 (0.217–1.158) | 0.106 | 0.886 (0.295–2.660) | 0.830 | |
| CCI | 0.846 (0.596–1.199) | 0.347 | 1.122 (0.764–1.648) | 0.558 | |
| Pulmonary function | |||||
| FEV1 | 1.020 (0.549–1.896) | 0.950 | 0.962 (0.473–1.959) | 0.916 | |
| FEV1%pred | 1.029 (1.005–1.054) | 0.019 | 1.033 (1.006–1.061) | 0.015 | |
| DLCO%pred | 1.013 (0.991–1.035) | 0.243 | 1.004 (0.979–1.029) | 0.766 | |
| Histology (non-SCC vs. SCC) | 1.995 (0.922–4.314) | 0.079 | 1.511 (0.618–3.698) | 0.366 | |
| Number of LNs removed | 0.962 (0.916–1.010) | 0.12 | 0.948 (0.893–1.006) | 0.078 | |
| Stations of LNs removed | 0.919 (0.640–1.320) | 0.647 | 0.749 (0.494–1.136) | 0.174 | |
| Pathological N stage | |||||
| N0 | Reference | Reference | |||
| N1 | 0.921 (0.260–3.264) | 0.898 | 0.790 (0.171–3.660) | 0.764 | |
| N2 | 3.192 (1.402–7.271) | 0.006 | 3.151 (1.247–7.961) | 0.015 | |
| Pathological stage | |||||
| I | Reference | Reference | |||
| II | 3.521 (1.203–10.305) | 0.022 | 1.747 (0.533–5.726) | 0.357 | |
| III | 4.329 (1.502–12.476) | 0.007 | 3.176 (1.063–9.489) | 0.039 | |
| VPI (present) | 2.149 (0.805–5.735) | 0.127 | 2.632 (0.955–7.255) | 0.061 | |
| LVI (present) | 1.987 (0.834–4.737) | 0.121 | 1.895 (0.686–5.233) | 0.218 | |
| Necrosis (present) | 0.370 (0.087–1.566) | 0.177 | 1.217 (0.405–3.660) | 0.726 | |
BMI, body mass index; CCI, Charlson Comorbidity Index; FEV1, forced expiratory volume in one second; DLCO, diffusing capacity of lung for carbon monoxide; SCC, squamous cell carcinoma; %pred, percentage predicted; LN, lymph node; VPI, visceral pleura invasion; LVI, lymphovascular invasion; HR, hazard ratio; CI, confidence interval; DFS, disease-free survival; OS, overall survival.
Univariate survival analyses (Table 3) demonstrated that gender (P=0.020), FEV1%pred (P=0.019), N2 stage (P=0.006), and higher pathological stage (II, P=0.022; III, P=0.007) were associated with worse DFS. As for OS, FEV1%pred (P=0.015), N2 stage (P=0.015) and higher pathological stage (III, P=0.039) was associated with worse OS. Furthermore, the risk factors with P<0.1 for DFS and OS in the univariable analyses were subsumed into the multivariable Cox analyses shown in Table 4. The hazard ratio (HR) of FEV1%pred was close to 1 for DFS and OS (DFS, HR, 1.028/1.027; OS, HR, 1.020/1.022), suggesting that it had little reference value for risk prediction. Since the pathological stage includes the N stage, pathological stage and N stage were separated and included in different multivariate Cox proportional hazards regression models. In the multivariate analysis including pathological stage and other risk factors, higher pathological stage [stage II: HR =3.297, 95% confidence interval (CI): 1.103 to 9.852, P=0.033; stage III: HR =3.561, 95% CI: 1.186 to 10.688, P=0.024] was identified as an independent risk factor for poorer DFS. Higher pathological stage (stage III: HR =3.346, 95% CI: 1.098 to 10.194, P=0.034) was also shown to be an independent risk factor for poor OS. In the multivariate analysis including N stage and other risk factors, N2 stage was found to be an independent risk factor for worse DFS (HR =2.789, 95% CI: 1.187 to 6.553, P=0.019) and worse OS (HR =2.957, 95% CI: 1.164 to 7.514, P=0.023).
Table 4
| Characteristics | DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI)a | Pa | HR (95% CI)b | Pb | HR (95% CI)c | Pc | HR (95% CI)d | Pd | ||
| Gender (female) | 2.243 (0.844–5.958) | 0.105 | 2.388 (0.879–6.485) | 0.088 | |||||
| FEV1%pred | 1.028 (1.003–1.053) | 0.025 | 1.027 (1.003–1.052) | 0.029 | 1.020 (0.995–1.045) | 0.123 | 1.022 (0.995–1.049) | 0.108 | |
| Histology (non-SCC vs. SCC) | 1.108 (0.481–2.551) | 0.810 | 1.188 (0.504–2.796) | 0.694 | |||||
| Number of LNs removed | 0.942 (0.884–1.004) | 0.068 | 0.950 (0.893–1.009) | 0.096 | |||||
| Pathological N stage | |||||||||
| N0 | Reference | Reference | |||||||
| N1 | 0.845 (0.236–3.019) | 0.795 | 0.719 (0.154–3.351) | 0.675 | |||||
| N2 | 2.789 (1.187–6.553) | 0.019 | 2.957 (1.164–7.514) | 0.023 | |||||
| Pathological stage | |||||||||
| I | Reference | Reference | |||||||
| II | 3.297 (1.103–9.852) | 0.033 | 1.824 (0.551–6.035) | 0.325 | |||||
| III | 3.561 (1.186–10.688) | 0.024 | 3.346 (1.098–10.194) | 0.034 | |||||
| VPI (presence) | 2.608 (0.865–7.859) | 0.089 | 2.179 (0.694–6.839) | 0.182 | |||||
a, factors of the multivariable Cox model: gender (female), FEV1%pred, histology (non-SCC vs. SCC), pathological stage; b, factors of the multivariable Cox model: gender (female), FEV1%pred, histology (non-SCC vs. SCC), pathological N stage; c, factors of the multivariable Cox model: FEV1%pred, Number of LNs removed, pathological stage; d, factors of the multivariable Cox model: FEV1%pred, Number of LNs removed, pathological N stage. FEV1, forced expiratory volume in one second; SCC, squamous cell carcinoma; %pred, percentage predicted; LN, lymph node; VPI, visceral pleura invasion; LVI, lymphovascular invasion; HR, hazard ratio; CI, confidence interval; DFS, disease-free survival; OS, overall survival.
Discussion
In this retrospective study, a series of 104 patients who underwent robot-assisted bronchial single sleeve lobectomy was reviewed to further reinforce the oncological feasibility of sleeve lobectomy via a robotic surgical system. We previously reported our technique and outcomes in 67 cases who underwent robotic single sleeve lobectomy (9) confirming the feasibility and low complication rate related to this procedures. To the best of our knowledge, this is the first report of long-term survival data on robot-assisted sleeve lobectomy.
Bronchial sleeve lobectomy has been shown to be technically feasible with superior postoperative outcomes and oncologic prognosis, and therefore, it has been widely considered as a treatment for centrally located NSCLC to avoid pneumonectomy (1-3). Bronchial sleeve lobectomy can ensure radical resection of the lesion while retaining more lung tissue. The current National Comprehensive Cancer Network (NCCN) guidelines suggest that sleeve lobectomy for lung-sparing anatomic resection is preferred over pneumonectomy, if anatomically appropriate and margin-negative resection is achieved (16). With the enormous advancements in minimally invasive technologies, including robotic-assisted thoracoscopic surgery (RATS) and video-assisted thoracoscopic surgery (VATS), these methods are now considered safe and feasible in bronchial sleeve resection compared with thoracotomy (10,11,17-19). Furthermore, compared to VATS, the three-dimensional magnified vision and EndoWrist technology of robotic surgical systems allow surgeons to operate more flexibly with the robotic surgical instruments, especially the suture operations in bronchial sleeve resection (5,9). Our previous research showed that, compared with VATS and open thoracotomy, robot-assisted sleeve lobectomy resulted in lower blood loss, earlier drainage tube removal, shorter surgical time, and a similar oncological prognosis (10). In the present study, the indications for use of robot-assisted sleeve lobectomy include tumor involving the origin of the lobar bronchus, direct invasion from metastatic LNs, and microscopic residual disease on the bronchial margin as shown by frozen sections after robotic standard lobectomy, and this is consistent with our previous studies (9,10).
In this study cohort, the 5-year DFS and OS rates were 67.9% and 73.0%, respectively. Reviewing all the cohort studies on robotic bronchial sleeve resection to date (4-8,11), this is the first study to report long-term survival results following robot-assisted bronchial single sleeve lobectomy. Our previous study showed that RATS had similar mid-term oncological outcomes compared with VATS or open thoracotomy for sleeve lobectomy (10). As illustrated in Table 5, recent studies on bronchial sleeve lobectomy or bronchoplasty showed that the 5-year DFS rates ranged from 44.7% to 62.9%, and the 5-year OS rates ranged from 37.5% to 72.2% (18-27). By comparison, the long-term survival rates of robot-assisted bronchial single sleeve lobectomy shown in this study are oncologically encouraging and adequate. The survival rates of a given study cohort are generally influenced by several characteristics, such as the proportion of higher pathological stages or LN metastasis, composition of histological types, the pathological features of tumor invasion, closer follow-up consultations, more aggressive postoperative adjuvant therapy and so on. While improvements in comprehensive treatment and progress in treatment philosophy can increase survival rates, the superior survival of our series might indicate the potential advantages of robotic surgery.
Table 5
| Authors | Publication year | Surgical procedure | Number of patients | Surgical resection | 5-year DFS (%) | 5-year OS (%) |
|---|---|---|---|---|---|---|
| Current study | – | RATS | 104 | Single sleeve lobectomy | 67.9 | 73.0 |
| Chen et al. (20) | 2021 | Thoracotomy and VATS | 665 | Sleeve lobectomy | 56.6 | 61.0 |
| Inci et al. (21) | 2020 | Thoracotomy | 106 | Standard sleeve lobectomy | – | 57.6 |
| 81 | Complex sleeve lobectomy | – | 56.2 | |||
| Yang et al. (19) | 2020 | Thoracotomy | 143 | Sleeve lobectomy | 59.0 | 59.6 |
| VATS | 44 | 54.6 | 56.1 | |||
| Gao et al. (18) | 2019 | Thoracotomy | 94 | Sleeve lobectomy | 44.7 | 61.7 |
| VATS | 54 | 59.3 | 72.2 | |||
| Maurizi et al. (22) | 2018 | Thoracotomy | 28 | Y-sleeve resections | 62.9 | 55.1 |
| Hong et al. (23) | 2018 | Thoracotomy | 63 | Extended sleeve lobectomy | 59.0 | 62.0 |
| 477 | Simple sleeve lobectomy | 57.0 | 69.0 | |||
| Nagayasu et al. (24) | 2016 | Thoracotomy | 100 | Bronchoplasty | – | 45.6–60.9 |
| Tagawa et al. (25) | 2016 | Thoracotomy | 151 | Sleeve lobectomy | – | 62.6 |
| Andersson et al. (26) | 2015 | Thoracotomy | 40 | Sleeve lobectomy | – | 37.5 |
| Zhao et al. (27) | 2015 | Thoracotomy | 161 | Bronchoplastic and broncho-arterioplastic | 48.2 | 53.4 |
RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; DFS, disease-free survival; OS, overall survival.
A recent national study using the U.S. National Cancer Data Base (NCDB) conducted by Mayne and colleagues (28) described the excellent long-term survival rates among patients who underwent sleeve resection as follows: the 5-year OS rate was 85% in 44 patients who had VTAS, and 79% in 166 patients who had open surgery (28). In Mayne’s study cohort, most patients (54%/57%) had pathological stage I disease, and more than half of the patients (55%) had carcinoid tumors (28), which are recognized as low-grade malignant tumors (29,30). In contrast, patients with lung SCC accounted for the vast majority of cases in our study cohort (71.2%), as well as in other retrospective studies (ranging from 67.9% to 82.5%) (17-19,23,24). Furthermore, there were only 2 (1.9%) patients with carcinoid tumor in our cohort. Therefore, we speculate that the different components of case series, especially the higher proportion of carcinoid tumors, may be the reasons for the excellent survival rate in Mayne’s study (28).
Analysis of the long-term outcomes in this report revealed that recurrence occurred in 26 (25.0%) patients. Among them, 10 (9.6%) patients had locoregional recurrence including LN and pulmonary recurrence, and 16 (15.4%) patients relapsed with distant metastasis. In recent studies, the recurrence rates after sleeve resection ranged from 24.0% to 43.2% (11,17-19,25). Therefore, the recurrence results of long-term follow-up in the present study demonstrated that robot-assisted bronchial single sleeve lobectomy may be adequate in oncological prognosis. Additionally, the tumor recurrence associated with surgical operations is manifested in recurrence along the suture line or around the bronchial anastomosis, which is mainly caused by insufficient bronchial resection or microscopic tumor residue. In our series, no endobronchial or perianastomotic recurrence occurred at the level of bronchial reconstruction in any of the patients, which supported the oncological reliability of the robotic surgery for bronchial sleeve lobectomy. The flexible and compact wristed instruments of the robotic surgical system can maximize the range of bronchial resection, making it easier to achieve a sufficiently safe margin in oncology. Therefore, the long-term survival and recurrence results of this study cohort indicate that robot-assisted bronchial single sleeve lobectomy can be performed by experienced surgeons without compromising oncologic prognosis.
Univariate and multivariate Cox analyses were performed to investigate the risk factors that impair survival. The results showed that higher pathological stage or N2 stage were independent prognostic factors for centrally located NSCLC patients who underwent robot-assisted bronchial single sleeve lobectomy, and this is consistent with previous investigations (10,13,17,21). Therefore, patients with stage III or N2 disease had a significantly worse survival, and poor DFS was observed in stage II and III patients. Moreover, there was no significant difference in the DFS rate between stage II and III cases, nor the OS rate between stage I and II patients. This may be due to the smaller sample size after grouping or stratification according to other pathological features affecting survival. Future studies involving a larger sample series, more detailed stratified analyses, or longer-term follow-up are warranted to confirm these results. In the present study, compared with N0 stage, N1 stage was not a significant prognostic factor in long-term survival, which was consistent with other studies (10,21,31). Therefore, patients with higher stage or N2 stage disease may have impaired survival and appropriate comprehensive treatments, including neoadjuvant and adjuvant therapy, may be required to improve the prognosis of such patients (32,33).
There were several limitations to this study. First, this study was a retrospective analysis from a single institution and the results may be influenced by selection bias. Second, the series of robot-assisted sleeve lobectomy cases were performed by a single established surgeon in our center, which limits the applicability of our experience to other institutions. Therefore, it is necessary to further verify these results in multicentered, prospective, randomized, controlled clinical trials with larger sample sizes.
In conclusion, robot-assisted bronchial single sleeve lobectomy could be an oncologically adequate procedure for patients with centrally located NSCLC. Further studies of comparative studies or high-quality randomized controlled trials are required.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: This study was funded by the Natural Science Foundation of Shandong Province (No. ZR2020MH234).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-298/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-298/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-298/coif). GV received honoraria from Ab Medica, Medtronic, MSD and Roche, which all outside the submitted work, and she is the Chair of Robotic working Group in European Society of Thoracic Surgeons (ESTS). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the medical ethics committee of the Affiliated Hospital of Qingdao University (No. QYFYWZLL 26574). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Li Z, Chen W, Xia M, et al. Sleeve lobectomy compared with pneumonectomy for operable centrally located non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2019;8:775-86. [Crossref] [PubMed]
- Shi W, Zhang W, Sun H, et al. Sleeve lobectomy versus pneumonectomy for non-small cell lung cancer: a meta-analysis. World J Surg Oncol 2012;10:265. [Crossref] [PubMed]
- Geraci TC, Ferrari-Light D, Wang S, et al. Robotic Sleeve Resection of the Airway: Outcomes and Technical Conduct Using Video Vignettes. Ann Thorac Surg 2020;110:236-40. [Crossref] [PubMed]
- Pan X, Gu C, Wang R, et al. Initial Experience of Robotic Sleeve Resection for Lung Cancer Patients. Ann Thorac Surg 2016;102:1892-7. [Crossref] [PubMed]
- Lin MW, Kuo SW, Yang SM, et al. Robotic-assisted thoracoscopic sleeve lobectomy for locally advanced lung cancer. J Thorac Dis 2016;8:1747-52. [Crossref] [PubMed]
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. [PubMed]
- Li C, Zhou B, Han Y, et al. Robotic sleeve resection for pulmonary disease. World J Surg Oncol 2018;16:74. [Crossref] [PubMed]
- Jiao W, Zhao Y, Qiu T, et al. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann Thorac Surg 2019;108:211-8. [Crossref] [PubMed]
- Qiu T, Zhao Y, Xuan Y, et al. Robotic sleeve lobectomy for centrally located non-small cell lung cancer: A propensity score-weighted comparison with thoracoscopic and open surgery. J Thorac Cardiovasc Surg 2020;160:838-846.e2. [Crossref] [PubMed]
- Gu C, Pan X, Chen Y, et al. Short-term and mid-term survival in bronchial sleeve resection by robotic system versus thoracotomy for centrally located lung cancer. Eur J Cardiothorac Surg 2018;53:648-55. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Qiu T, Zhao Y, Xuan Y, et al. Robotic-assisted double-sleeve lobectomy. J Thorac Dis 2017;9:E21-E25. [Crossref] [PubMed]
- Zhao Y, Jiao W, Ren X, et al. Left lower lobe sleeve lobectomy for lung cancer using the Da Vinci surgical system. J Cardiothorac Surg 2016;11:59. [Crossref] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines).Non-small cell lung cancer. Version 5 2021 Accessed June 15, 2021. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Xie D, Deng J, Gonzalez-Rivas D, et al. Comparison of video-assisted thoracoscopic surgery with thoracotomy in bronchial sleeve lobectomy for centrally located non-small cell lung cancer. J Thorac Cardiovasc Surg 2021;161:403-413.e2. [Crossref] [PubMed]
- Gao HJ, Jiang ZH, Gong L, et al. Video-Assisted Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity Matched Analysis. Ann Thorac Surg 2019;108:1072-9. [Crossref] [PubMed]
- Yang Y, Mei J, Lin F, et al. Comparison of the Short- and Long-term Outcomes of Video-assisted Thoracoscopic Surgery versus Open Thoracotomy Bronchial Sleeve Lobectomy for Central Lung Cancer: A Retrospective Propensity Score Matched Cohort Study. Ann Surg Oncol 2020;27:4384-93. [Crossref] [PubMed]
- Chen J, Soultanis KM, Sun F, et al. Outcomes of sleeve lobectomy versus pneumonectomy: A propensity score-matched study. J Thorac Cardiovasc Surg 2021;162:1619-1628.e4. [Crossref] [PubMed]
- Inci I, Benker M, Çitak N, et al. Complex sleeve lobectomy has the same surgical outcome when compared with conventional lobectomy in patients with lung cancer. Eur J Cardiothorac Surg 2020;57:860-6. [Crossref] [PubMed]
- Maurizi G, Ciccone AM, Vanni C, et al. Reimplantation of the upper lobe bronchus after lower sleeve lobectomy or bilobectomy: long-term results. Eur J Cardiothorac Surg 2018;53:1180-5. [Crossref] [PubMed]
- Hong TH, Cho JH, Shin S, et al. Extended sleeve lobectomy for centrally located non-small-cell lung cancer: a 20-year single-centre experience. Eur J Cardiothorac Surg 2018;54:142-8. [Crossref] [PubMed]
- Nagayasu T, Yamasaki N, Tsuchiya T, et al. The evolution of bronchoplasty and broncho-angioplasty as treatments for lung cancer: evaluation of 30 years of data from a single institution. Eur J Cardiothorac Surg 2016;49:300-6. [Crossref] [PubMed]
- Tagawa T, Iwata T, Nakajima T, et al. Evolution of a Lung-Sparing Strategy with Sleeve Lobectomy and Induction Therapy for Non-small Cell Lung Cancer: 20-Year Experience at a Single Institution. World J Surg 2016;40:906-12. [Crossref] [PubMed]
- Andersson SE, Rauma VH, Sihvo EI, et al. Bronchial sleeve resection or pneumonectomy for non-small cell lung cancer: a propensity-matched analysis of long-term results, survival and quality of life. J Thorac Dis 2015;7:1742-8. [PubMed]
- Zhao LL, Zhou FY, Dai CY, et al. Prognostic analysis of the bronchoplastic and broncho-arterioplastic lobectomy of non-small cell lung cancers-10-year experiences of 161 patients. J Thorac Dis 2015;7:2288-99. [PubMed]
- Mayne NR, Darling AJ, Raman V, et al. Perioperative Outcomes and 5-year Survival After Open versus Thoracoscopic Sleeve Resection for Lung Cancer. Semin Thorac Cardiovasc Surg 2021;33:522-30. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Anile M, Diso D, Rendina EA, et al. Bronchoplastic procedures for carcinoid tumors. Thorac Surg Clin 2014;24:299-303. [Crossref] [PubMed]
- Berry MF, Worni M, Wang X, et al. Sleeve lobectomy for non-small cell lung cancer with N1 nodal disease does not compromise survival. Ann Thorac Surg 2014;97:230-5. [Crossref] [PubMed]
- Booth CM, Shepherd FA, Peng Y, et al. Adoption of adjuvant chemotherapy for non-small-cell lung cancer: a population-based outcomes study. J Clin Oncol 2010;28:3472-8. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol 2019;37:537-46. [Crossref] [PubMed]


