
The efficacy of anlotinib as third-line treatment for non-small cell lung cancer by EGFR mutation status: a subgroup analysis of the ALTER0303 randomized phase 3 study
Introduction
Over the past decade, great progress has been achieved in the treatment of advanced non-small cell lung cancer (NSCLC). Targeted therapy determined by individual molecular profiles, including immunotherapy and anti-angiogenesis therapy, has improved survival. However, most patients will develop progressive disease after response to first- and second-line therapy, while the subsequent treatment is less hopeful and a clear standard has not been established, and there is an unmet need to improve treatment for this population.
Angiogenesis is a complex process that plays an important role in sustaining the tumor microenvironment, tumor growth, and metastatic dissemination, involving both pro-angiogenic and anti-angiogenic molecules. The vascular endothelial growth factor (VEGF) family members play a key role in angiogenesis, among which VEGFA is the main mediator of tumor-associated angiogenesis. Of the VEGF receptors that are crucial for the transmission of angiogenic signals, VEGFR-2 is the major mediator of the mitogenic, angiogenic, and permeability effects of VEGF (1). Several other pro-angiogenic factors also impact tumor angiogenesis, including platelet-derived growth factor (PDGF) and fibroblast growth factor (FGF). Cancer cells can express and secret PDGFβ and FGF-2 at a high level, which assists in stimulating angiogenesis (2).
Treatment by targeting tumor angiogenesis has been approached through several methods, including monoclonal antibodies that block VEGF-VEGFR binding, VEGFR tyrosine kinase inhibitors (TKIs), and VEGF Trap (3). Bevacizumab and ramucirumab, which are monoclonal antibodies targeting VEGF and VEGFR, respectively, have led to improved overall survival (OS) for NSCLC when added to standard first- and second-line chemotherapy. However, only about one third of patients respond to anti-VEGF antibodies (4,5). Previously, VEGFR TKIs failed to show significant prolongation of OS either in combination with chemotherapy as first- and second-line therapy, or in comparison with placebo as third- and fourth-line therapy, including vandetanib, sorafenib, sunitinib, axitinib, and cediranib (6).
Anlotinib is a novel multi-target TKI that inhibits VEGFR2/3, FGFR1-4, PDGFR α/β, c-Kit, and Ret, among others (7). In a preclinical study, the inhibition of VEGFR2, PDGFRβ, and FGFR1 by anlotinib was stronger than sunitinib and sorafenib (8). Anlotinib showed higher selectivity for VEGFR family members than other VEGFR TKIs, which may indicate milder toxicity (9). In the ALTER0302 trial, a phase 2 study of anlotinib as a third-line treatment for patients with advanced NSCLC compared with placebo, progression-free survival (PFS) was prolonged significantly in the anlotinib group regardless of EGFR mutation status in the post-hoc subgroup analysis (10). In the ALTER0303 trial, anlotinib significantly improved PFS and OS as a third-line and subsequent treatment for NSCLC patients regardless of EGFR mutation status (11). In China, EGFR mutations are found in up to 45% of NSCLC patients (12). In previous studies, the response rates of chemotherapy in patients with and without EGFR mutation status were different (13), and frontline EGFR TKI may reduce the efficacy of chemotherapy in patients with EGFR mutation (14). The previous treatments of patients with and without EGFR mutation are distinct, and we analyzed the efficacy and safety of anlotinib in these two subpopulations in the ALTER0303 trial. We present the following article in accordance with the TREND reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-320/rc).
Methods
Study design and procedures
ALTER0303 was a randomized, double-blind, placebo-controlled phase 3 study (NCT02388919), which has been published (11). The study was conducted in accordance with Declaration of Helsinki (as revised in 2013) and Good Clinical Practice requirements. The study was approved by the institutional ethics board of each site. All the patients provided written informed consent before study entry.
The main inclusion criteria included: aged 18–75 years, histologically or cytologically confirmed NSCLC, at least 2 lines of chemotherapy for patients without driver mutation, and at least 1 line of chemotherapy and 1 line of TKI therapy for patients with known driver mutations. Subjects were randomized in a 2:1 ratio with a block randomization scheme (block size of 4) to receive oral anlotinib 12 mg/d or placebo on day 1–14 of a 21-day cycle. The primary endpoint was OS, the secondary endpoints included progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR) and safety. EGFR mutation status was one of the pre-stratification factors. Patients should have formal report of EGFR and ALK status, those who did not have report should provide specimens for central detection. Tumor assessment was conducted with computed tomography every 2 cycles, follow-up was done every 8 weeks until death or the data cutoff date. The efficacy of treatment was assessed by RECIST v1.1 and treatment-related adverse events were graded according to NCI CTCAE v5.5.
We compared the outcomes according to potential covariates including prior duration of targeted therapy, the duration of EGFR TKI treatment, and the time since the start of prior therapy (TSPT) in patients assessed by EGFR mutation status. We identified 18 months as the cut-off point of TSPT, as it was the median time from the start of first-line treatment to study entry. Eight months was identified as the cut-off point of time to progression with targeted therapy as it was the median treatment duration of EGFR TKI.
Statistical analysis
We used the Kaplan-Meier estimates method to assess the median PFS and OS with 95% confidence intervals (CIs). Differences in survival were assessed using the log-rank test. The proportional hazards (Cox) model was used to estimate hazard ratios (HRs) for PFS and OS with 95% CIs. To verify the relationship between EGFR mutation status and treatment efficacy, an interaction term of EGFR mutation status and the intervention of anlotinib or placebo was conducted using multiple regression model. All statistical tests were two sided, and P<0.05 was considered to be statistically significant. All analyses were performed using SAS software, version 9.4.
Results
Characteristics of patients according to EGFR mutation status
Of the 437 randomized patients, 138 (31.6%) were EGFR mutation positive, including 93 in the anlotinib group and 45 in the placebo group, while 299 (68.4%) were EGFR mutation negative, including 201 in the anlotinib group and 98 in the placebo group. Baseline characteristics were balanced between treatment arms in both the EGFR mutation positive and negative subgroups. In patients with EGFR mutation, the proportion of female and adenocarcinoma was higher, which are known clinical factors associated with higher EGFR mutation frequency (Table 1).
Table 1
| Characteristics | EGFR M+, n (%) | EGFR M−, n (%) | |||
|---|---|---|---|---|---|
| Anlotinib (n=93) | Placebo (n=45) | Anlotinib (n=201) | Placebo (n=98) | ||
| Gender | |||||
| Male | 42 (45.2) | 23 (51.1) | 146 (72.6) | 74 (75.5) | |
| Female | 51 (54.8) | 22 (48. 9) | 55 (27.4) | 24 (24.5) | |
| Age, years | |||||
| ≤60 | 55 (59.1) | 31 (68.9) | 98 (48.7) | 59 (60.2) | |
| 61–69 | 34 (36.6) | 11 (24.4) | 91 (45.3) | 30 (30.6) | |
| ≥70 | 4 (4.3) | 3 (6.7) | 12 (6.0) | 9 (9.2) | |
| Pathological diagnosis | |||||
| Adeno-carcinoma | 85 (91.4) | 43 (95.6) | 143 (71.1) | 65 (66.3) | |
| Squamous | 6 (6.5) | 2 (4.4) | 47 (23.4) | 31 (31.6) | |
| Others | 2 (2.2) | 0 (0.0) | 11 (5.5) | 2 (2.1) | |
| Clinical stage | |||||
| IIIB | 2 (2.1) | 3 (6.7) | 13 (6.5) | 4 (4.1) | |
| IV | 91 (97.9) | 42 (93.3) | 186 (92.5) | 94 (95.9) | |
| Others | 0 (0.0) | 0 (0.0) | 2 (1.0) | 0 0.0)) | |
| Number of metastases | |||||
| >3 | 44 (47.3) | 23 (51.1) | 79 (39.3) | 39 (39.8) | |
| ≤3 | 49 (52.7) | 22 (48.9) | 122 (60.7) | 59 (60.2) | |
| Lines of prior chemotherapy | |||||
| 2 | 55 (59.1) | 26 (57.8) | 112 (55.7) | 52 (53.1) | |
| 3 | 34 (36.6) | 19 (42.2) | 89 (44.3) | 46 (46.9) | |
| 1 | 4 (4.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Prior targeted therapy | |||||
| No | 2 (2.2) | 3 (6.7) | 134 (66.7) | 71 (72.5) | |
| Yes | 91 (97.8) | 42 (93.3) | 67 (33.3) | 27 (27.5) | |
| Prior radiotherapy | |||||
| No | 59 (63.4) | 20 (44.4) | 117 (58.2) | 58 (59.2) | |
| Yes | 34 (36.6) | 25 (55.6) | 84 (41.8) | 40 (40.8) | |
| ECOG PS | |||||
| 0 | 22 (23.7) | 6 (13.3) | 37 (18.4) | 16 (16.3) | |
| 1 | 71 (76.3) | 39 (86.7) | 162 (80.6) | 81 (82.7) | |
| 2 | 0 (0.0) | 0 (0.0) | 2 (1.0) | 1 (1.0) | |
| Smoking history | |||||
| Once or current smoker | 1 (1.1) | 2 (4.4) | 12 (6.0) | 8 (8.2) | |
| None-smoker | 92 (98.9) | 43 (95.6) | 189 (94.0) | 90 (91.8) | |
| ALK status | |||||
| Negative | 93 (100.0) | 45 (100.0) | 193 (96.0) | 95 (97.0) | |
| Positive | 0 (0.0) | 0 (0.0) | 5 (2.5) | 2 (2.0) | |
| Unknown | 0 (0.0) | 0 (0.0) | 3 (1.5) | 1 (1.0) | |
EGFR, epidermal growth factor receptor; M+, mutation positive; M−, mutation negative; ECOG PS, eastern cooperative oncology group performance status; ALK, anaplastic lymphoma kinase.
The efficacy of anlotinib in patients with and without EGFR mutation
In the anlotinib group, the PFS for patients with and without EGFR mutation was 5.6 and 5.4 months (HR 1.00; 95% CI: 0.75–1.34, P=1.000), the OS was 10.7 and 8.9 months (HR 0.69; 95% CI: 0.50–0.95, P=0.021) respectively; while in the placebo group, the PFS for patients with and without EGFR mutation was 0.8 and 1.6 months (HR 1.53; 95% CI: 1.03–2.26, P=0.033), the OS was 6.3 and 6.5 months respectively (HR 0.87; 95% CI: 0.57–1.35, P=0.541).
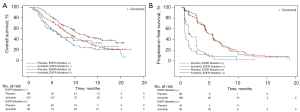
In patients with EGFR mutation, the OS was 10.7 and 6.3 months (HR 0.59; 95% CI: 0.38–0.94, P=0.025) for anlotinib and placebo, respectively. The PFS was 5.6 and 0.8 months (HR 0.21; 95% CI: 0.13–0.32, P<0.0001) for anlotinib and placebo, respectively. The ORR was 7.5% for anlotinib and 0% for placebo, while the DCR was 82.8% for anlotinib and 20.0% for placebo. In patients without EGFR mutation, the OS was 8.9 and 6.5 months (HR 0.73; 95% CI: 0.55–0.97, P=0.029) for anlotinib and placebo, respectively. The PFS was 5.4 and 1.6 months (HR 0.29; 95% CI: 0.22–0.39, P<0.0001) for anlotinib and placebo, respectively. In the multiple Cox regression analysis, the interaction term of OS and PFS was 0.1064 and 0.4485 respectively (Tables S1-S4). The ORR was 10.0% for anlotinib and 1.0% for placebo, while the DCR was 80.1% for anlotinib and 44.9% for placebo (Figure 1A,1B).
The effect of previous targeted therapy on survival in patients with EGFR mutation
Among the 138 patients with EGFR mutation, 133 received EGFR TKIs before study entry (91/93 in the anlotinib group and 42/45 in the placebo group). A total of 27 (20.3%) received EGFR TKIs as first-line therapy, while 106 (79.7%) received EGFR TKIs as second-line therapy. The proportion of patients who received EGFR TKIs as first-line therapy in the anlotinib group was lower (17.6% vs. 26.2%). The most frequently used EGFR TKIs were gefitinib, erlotinib, and icotinib. For patients who received TKIs as first-line therapy (16/93 in the anlotinib group, 11/45 in the placebo group), the PFS was 4.3 months for anlotinib and 0.8 months for placebo (HR 0.32; 95% CI: 0.11–0.91, P=0.032), while the OS was 11.1 months for anlotinib and 3.5 months for placebo (HR 0.31; 95% CI: 0.11–0.87, P=0.026; Figure S1A,S1B). For those who received EGFR TKIs as second-line therapy (75/93 in the anlotinib group, 31/45 in the placebo group), the PFS was 5.6 months for anlotinib and 0.9 months for placebo (HR 0.2; 95% CI: 0.12–0.34, P<0.0001), while the OS was 10.7 months for anlotinib and 7.0 months for placebo (HR 0.69; 95% CI: 0.4–1.18, P=0.17; Figure S1C,S1D).
The median treatment duration of previous targeted therapy was 8 months. Patients who progressed from EGFR TKIs within 8 months (30 in the anlotinib group, 11 in the placebo group) had significantly longer PFS (4.5 vs. 0.7 months, HR 0.07; 95% CI: 0.02–0.26, P<0.0001) and OS (10.0 vs. 3.4 months, HR 0.39; 95% CI: 0.17–0.9, P=0.03) with anlotinib compared with placebo (Figure S2A,S2B). For those who progressed from EGFR TKIs exceeding 8 months (61 in the anlotinib group, 31 in the placebo group), the PFS with anlotinib was longer than with placebo (5.6 vs. 1.1 months; HR 0.24; 95% CI: 0.14–0.4, P<0.0001), while no significant difference in OS was found (12.9 vs. 7.0 months, HR 0.62; 95% CI: 0.35–1.09, P=0.1; Figure S2C,S2D).
The effect of TSPT on survival
The median time for patients from the start of prior treatment to study entry was 18 months. In patients with EGFR mutation whose TSPT ≤18 months (28 in the anlotinib group, 14 in the placebo group), the PFS (4.3 vs. 0.8 months, HR 0.18; 95% CI: 0.08–0.42, P<0.0001) was improved significantly with anlotinib compared with placebo, while the OS (6.6 vs. 4.4 months, HR 0.58; 95% CI: 0.27–1.28, P=0.18) was improved without a significant difference. In patients without EGFR mutation whose TSPT ≤18 months (110 in the anlotinib group, 61 in the placebo group), the PFS (4.2 vs. 1.4 months, HR 0.29; 95% CI: 0.19–0.42, P<0.0001) was improved significantly with anlotinib compared with placebo, while no difference in OS was found (7.3 and 6.3 months, HR 0.77; 95% CI: 0.54–1.1, P=0.15; Figure S3A,S3B).
In patients with EGFR mutation whose TSPT>18 months (65 in the anlotinib group, 31 in the placebo group), the PFS (5.6 vs. 0.8 months, HR 0.22; 95% CI: 0.13–0.36, P<0.0001) and OS (15.8 vs. 7.0 months, HR 0.55; 95% CI: 0.31–0.99, P=0.043) were improved with anlotinib. In patients without EGFR mutation whose TSPT >18 months (91 in the anlotinib group, 37 in the placebo group), the PFS was improved (5.8 vs. 2.8 months, HR 0.3; 95% CI: 0.19–0.48, P<0.0001) with anlotinib compared with placebo, while the OS (10.5 vs. 8.0 months, HR 0.79; 95% CI: 0.48–1.3, P=0.36) was prolonged without significance (Figure S3C,S3D).
Safety
Treatment-related adverse events (TRAEs) were more frequently in patients treated with anlotinib than placebo in both the subgroups, such as hypertension (64.5% vs. 11.1% in patients with EGFR mutation, 64.7% vs. 15.3% in patients without EGFR mutation), elevated thyroglobulin (52.7% vs. 0, 40.8% vs. 6.1%), hand and foot syndrome (48.4% vs. 8.9%, 40.8% vs. 9.2%), elevated triglyceride (41.9% vs. 15.6%, 37.8% vs. 20.4%), proteinuria (26.9% vs. 8.9%, 25.9% vs. 15.3%), and rash (7.5% vs. 4.4%, 13.4% vs. 6.1%). The most common grade 3–4 TRAEs with anlotinib in patients with and without EGFR mutation were hypertension (9.7%, 14.9%) and elevated triglyceride (3.2%, 2.0%), which were manageable. Most of the TRAEs with anlotinib were similar in patients with and without EGFR mutation, while elevated thyroglobulin and hand and foot syndrome occurred more frequently in patients with EGFR mutation (52.7% vs. 40.8%). Rash occurred more frequently in patients without EGFR mutation (7.5% vs. 13.4%) (Table 2).
Table 2
| Event | EGFR M+ (n=138) (%) | EGFR M− (n=299) (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anlotinib (n=93) | Placebo (n=45) | Anlotinib (n=201) | Placebo (n=98) | ||||||||
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | ||||
| Hypertension | 60 (64.5) | 9 (9.7) | 5 (11.1) | 0 | 130 (64.7) | 30 (14.9) | 15 (15.3) | 0 | |||
| Elevated thyroglobulin | 49 (52.7) | 0 | 0 | 0 | 82 (40.8) | 1 (0.5) | 6 (6.1) | 0 | |||
| Hand and foot syndrome | 45 (48.4) | 2 (2.2) | 4 (8.9) | 0 | 82 (40.8) | 9 (4.5) | 9 (9.2) | 0 | |||
| Elevated triglyceride | 39 (41.9) | 3 (3.2) | 7 (15. 6) | 0 | 75 (37.8) | 4 (2.0) | 20 (20.4) | 0 | |||
| Proteinuria | 25 (26.9) | 2 (2.2) | 4 (8.9) | 1 (2.2) | 52 (25.9) | 5 (2.5) | 15 (15.3) | 0 | |||
| Rash | 7 (7.5) | 0 | 2 (4.4) | 0 | 27 (13.4) | 0 | 6 (6.1) | 1 (1.0) | |||
EGFR, epidermal growth factor receptor; M+, mutation positive; M−, mutation negative.
Discussion
In these subgroup analyses, anlotinib demonstrated significant OS and PFS benefits in patients with and without EGFR mutation who had failed at least 2 lines of therapy. During the study recruitment, most patients with EGFR mutation still received chemotherapy as first-line therapy, due to the relatively low EGFR mutation detection rate at diagnosis several years ago. Osimertinib was not approved at that time, and patients who progressed from first-line EGFR TKIs usually received chemotherapy as second-line treatment without T790M detection. Only 19.6% of patients with EGFR mutation received EGFR TKIs as first-line therapy in this study. Approximately 60% of patients with EGFR mutation who received first-generation EGFR TKIs will develop a secondary T790M mutation on progression (15), and osimertinib has been approved as a standard second-line therapy for this population (16). For patients without T790M mutation or those with T790M mutation who do not respond to osimertinib, chemotherapy remains the primary choice, but the clinical benefit from chemotherapy may be reduced substantially after EGFR TKI treatment (14). Several anti-PD-1/PD-L1 antibodies have been approved for NSCLC as second- and further-line therapies (17), but a low percentage of patients with EGFR mutations benefit from immunotherapy (18). Anti-angiogenic agents, ramucirumab and nintedanib, showed improved survival in combination with docetaxel as a second-line therapy (5,19), but neither is approved in China.
For patients with EGFR mutation who had disease progression after EGFR TKI treatment and chemotherapy, anlotinib achieved a PFS of 5.6 months and an OS of 10.7 months. For patients without EGFR mutation who progressed from 2 lines of chemotherapy, anlotinib achieved a PFS of 5.4 months and an OS of 8.9 months, which was consistent with the results in a previous phase 2 study (10). In previous second-line studies, the PFS was 2.3–3.9 months and the OS was 9.2–13.8 months with immunotherapy, the PFS and OS were 3.4–4.5 and 10.1–10.5 months respectively with the combination of anti-angiogenic agents and docetaxel (5,17,19). This is the first VEGFR TKI that prolonged OS significantly as a single agent in the third-line setting, and the results of OS and PFS were promising and comparable with those in other second-line studies. The stronger effect on VEGF receptors than other VEGF TKIs, as well as the optimal effect on FGFR1-4 and PDGFR α/β which contribute to the resistance of anti-VEGF treatment, may explain the favorable treatment effect of anlotinib (20).
In our study, the prognosis of patients with EGFR mutation might be worse, for the PFS and OS were shorter than those without EGFR mutation in the placebo group. While in the anlotinib group, the OS of patients with EGFR mutation was significantly improve, indicating that patients with EGFR mutation might benefit more from anti-angiogenic therapy than those without EGFR mutation. It was reported the activation of EGFR can upregulate the expression of VEGF and VEGFR-1, activate VEGFR and promote angiogenesis. The dependence of EGFR mutant tumors on VEGF may explain our results (21).
In this analysis, the benefit of PFS and OS with anlotinib was found to be consistent in both patients with and without EGFR mutation. The pathological type of most patients with EGFR mutation was adenocarcinoma. In the LUME-Lung 1 study, a significant OS benefit (12.6 vs. 10.3 months) was demonstrated in the docetaxel plus nintedanib group compared with the docetaxel plus placebo group in adenocarcinoma patients but not in the total study population, indicating the efficacy of anti-angiogenic therapy for non-squamous NSCLC might be better than that of squamous NSCLC (19). In the ZEPHYER study, a greater benefit in PFS was suggested for vandetanib-treated patients with EGFR mutation compared with those without mutation (22). In the MISSION study, OS was similar in the whole population, while among the 89 patients with EGFR mutations, both OS (13.9 vs. 6.5 m) and PFS (2.7 vs. 1.4 m) were significantly improved with sorafenib compared with placebo (23). These results also suggest that EGFR mutation status may correlate with the treatment effect of VEGFR TKIs. There was previous evidence that mutant EGFR may enhance VEGF expression in lung cancers, suggesting that different angiogenic mechanisms might exist between EGFR mutation positive and negative tumors (24).
The LUME-Lung 1 and REVEL studies demonstrated survival advantages for nintedanib and ramucirumab plus docetaxel in advanced NSCLC patients with TSPT <9 months, suggesting that patients with a poorer prognosis may benefit more from the addition of anti-angiogenic therapy (18,25). Meanwhile, the improvement of OS in patients with EGFR mutation who progressed from EGFR TKIs within 8 months was significant with anlotinib. Tumors of greater invasiveness are more likely to depend on new blood vessels and benefit more from anti-angiogenic therapy. FGFR overexpression is mechanism of resistance to both EGFR-TKIs and cytotoxic chemotherapy, which is likely why the anlotinib benefit was consistent in patients whose tumors harbor EGFR mutations regardless of whether they were most recently treated with chemotherapy or an EGFR-TKI (26,27). This is also the basis for an ongoing study combining osimertinib and anlotinib for the first-line treatment of patient with advanced EGFR mutated NSCLC (28,29).
In our study, the benefit with anlotinib was consistent in subgroups, independent of EGFR mutation status and previous treatment. With the rapid progress in immunotherapy, checkpoint inhibitors have been applied as first-line therapy, alone or in combination with chemotherapy (30), which may affect the subsequent treatment choice of immunotherapy or chemotherapy. VEGF receptors are present in stromal cells and endothelial cells of the tumor microenvironment, and the action of anti-angiogenic agents on normal cells with a stable genome may be less affected by previous therapy. The ORRs were 7.5% and 10% with anlotinib in patients with and without EGFR mutation, respectively. The DCRs were 82.3% and 80.1% with anlotinib in patients with and without EGFR mutation, respectively, indicating that most patients had stable disease with anlotinib. Anti-VEGF therapy has primarily cytostatic effects and can normalize the tumor vasculature and have substantial systemic effects such as modulation of circulating pro-angiogenic and proinflammatory cytokines and cells, which stabilize the tumor size instead of shrinking it (31).
The safety profile of anlotinib was acceptable and favorable, similar to that described in previous studies (9,10). The most commonly reported TRAEs were typical VEGFR-inhibition related events and were similar in patients with and without EGFR mutation. The mild safety profile might establish the basis for the future combination of anlotinib with other EGFR TKIs such as osimertinib in patients with EGFR mutation, or combination with chemotherapy and immunotherapy in patients without EGFR mutation. The treatment of third-line EGFR mutated lung cancer continues to evolve and treatment strategies in this group now include anlotinib, docetaxel and EGFR-TKI monotherapy rechallenge with additional emerging therapies such as patritumab deruxtecan and amivantamab (32,33).
The present study had limitations. The standards for first and second-line therapy were altered in both EGFR mutation positive and negative patients. Most EGFR mutation positive patients received first-generation TKIs as second-line therapy in our study. At the start of the study, osimertinib was not approved in China, and patients who progressed from the first-generation TKI were not required to undergo T790M testing and most of them received chemotherapy subsequently without known T790 mutation status, which does not comply with the current guidelines. Likewise, checkpoint inhibitors were not approved during the study, and the efficacy of anlotinib after immunotherapy could not be evaluated. The sample size was small in subgroups, and it was difficult to draw firm conclusions in specific populations. The control arm was placebo, and additional studies are necessary to compare anlotinib with other approved subsequent treatments. Further studies will be conducted to evaluate anlotinib as a subsequent therapy in different populations.
Conclusions
In conclusion, anlotinib offers a third-line treatment option for patients with advanced NSCLC, regardless of EGFR mutation status and previous therapy strategies.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group. We thank all the participating patients and their families, the clinical trial team, and all the study personnel.
Funding: ALTER0303 was funded by Chia-Tai Tianqing Pharmaceutical Group Co., Ltd. The funder had no role in the study design, data collection and analysis.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-320/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-320/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-320/coif). All authors report this study was funded by Chia-Tai Tianqing Pharmaceutical Group Co., Ltd. DB reports that AstraZeneca has paid him for speakers’ fees and honoraria, but all funds went to his institution to support research. FT reports research grant from Boehringer Ingelheim Japan, Ono Pharmaceutical, Taiho Pharmaceutical, Chugai Pharmaceutical. FT also reports payment for lectures from MSD, Bristol-Meyers Squibb, Boehringer Ingelheim Japan, Ono Pharmaceutical, Johnson & Johnson, Covidien Japan, Taiho Pharmaceutical, Astra Zeneca, Chugai Phamaceutical. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of each site. The informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669-76. [Crossref] [PubMed]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005;438:967-74. [Crossref] [PubMed]
- Hall RD, Le TM, Haggstrom DE, et al. Angiogenesis inhibition as a therapeutic strategy in non-small cell lung cancer (NSCLC). Transl Lung Cancer Res 2015;4:515-23. [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665-73. [Crossref] [PubMed]
- Manzo A, Montanino A, Carillio G, et al. Angiogenesis Inhibitors in NSCLC. Int J Mol Sci 2017;18:2021. [Crossref] [PubMed]
- Sun Y, Niu W, Du F, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol 2016;9:105. [Crossref] [PubMed]
- Lin B, Song X, Yang D, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene 2018;654:77-86. [Crossref] [PubMed]
- Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci 2018;109:1207-19. [Crossref] [PubMed]
- Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer 2018;118:654-61. [Crossref] [PubMed]
- Han B, Li K, Wang Q, et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol 2018;4:1569-75. Erratum in: JAMA Oncol 2018;4:1625. [Crossref] [PubMed]
- Han B, Tjulandin S, Hagiwara K, et al. EGFR mutation prevalence in Asia-Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non-adenocarcinoma histology: The IGNITE study. Lung Cancer 2017;113:37-44. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-57. [Crossref] [PubMed]
- Zeng Z, Yan HH, Zhang XC, et al. Reduced chemotherapy sensitivity in EGFR-mutant lung cancer patient with frontline EGFR tyrosine kinase inhibitor. Lung Cancer 2014;86:219-24. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Cortinovis D, Gregorc V, Migliorino MR, et al. New perspectives in the second-line treatment of non squamous NSCLC patients: Results from a large Italian Lung Cancer Working Group. Crit Rev Oncol Hematol 2017;109:35-41. [Crossref] [PubMed]
- Bylicki O, Paleiron N, Margery J, et al. Targeting the PD-1/PD-L1 Immune Checkpoint in EGFR-Mutated or ALK-Translocated Non-Small-Cell Lung Cancer. Target Oncol 2017;12:563-9. [Crossref] [PubMed]
- Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014;15:143-55. [Crossref] [PubMed]
- Kerbel RS. Tumor angiogenesis. N Engl J Med 2008;358:2039-49. [Crossref] [PubMed]
- Langer C, Soria JC. The role of anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapies in the treatment of non-small-cell lung cancer. Clin Lung Cancer 2010;11:82-90. [Crossref] [PubMed]
- Lee JS, Hirsh V, Park K, et al. Vandetanib Versus placebo in patients with advanced non-small-cell lung cancer after prior therapy with an epidermal growth factor receptor tyrosine kinase inhibitor: a randomized, double-blind phase III trial (ZEPHYR). J Clin Oncol 2012;30:1114-21. [Crossref] [PubMed]
- Paz-Ares L, Hirsh V, Zhang L, et al. Monotherapy Administration of Sorafenib in Patients With Non-Small Cell Lung Cancer (MISSION) Trial: A Phase III, Multicenter, Placebo-Controlled Trial of Sorafenib in Patients with Relapsed or Refractory Predominantly Nonsquamous Non-Small-Cell Lung Cancer after 2 or 3 Previous Treatment Regimens. J Thorac Oncol 2015;10:1745-53. [Crossref] [PubMed]
- Hung MS, Chen IC, Lin PY, et al. Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol Lett 2016;12:4598-604. [Crossref] [PubMed]
- Reck M, Paz-Ares L, Bidoli P, et al. Outcomes in patients with aggressive or refractory disease from REVEL: A randomized phase III study of docetaxel with ramucirumab or placebo for second-line treatment of stage IV non-small-cell lung cancer. Lung Cancer 2017;112:181-7. [Crossref] [PubMed]
- Lian Z, Du W, Zhang Y, et al. Anlotinib can overcome acquired resistance to EGFR-TKIs via FGFR1 signaling in non-small cell lung cancer without harboring EGFR T790M mutation. Thorac Cancer 2020;11:1934-43. [Crossref] [PubMed]
- Raoof S, Mulford IJ, Frisco-Cabanos H, et al. Targeting FGFR overcomes EMT-mediated resistance in EGFR mutant non-small cell lung cancer. Oncogene 2019;38:6399-413. [Crossref] [PubMed]
- He Z, Lin J, He Y, et al. The combination of EGFR-TKIs and anlotinib as a first-line therapy for EGFR-mutant advanced non-small cell lung cancer: A multicenter, single-arm, phase II clinical trial. J Clin Oncol 2021;39:abstr 9030.
- Zhou B, Gong Q, Li B, et al. Clinical outcomes and safety of osimertinib plus anlotinib for patients with previously treated EGFR T790M-positive NSCLC: A retrospective study. J Clin Pharm Ther 2022;47:643-51. [Crossref] [PubMed]
- Martinez P, Peters S, Stammers T, et al. Immunotherapy for the First-Line Treatment of Patients with Metastatic Non-Small Cell Lung Cancer. Clin Cancer Res 2019;25:2691-8. [Crossref] [PubMed]
- Crabb SJ, Patsios D, Sauerbrei E, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol 2009;27:404-10. [Crossref] [PubMed]
- Jänne PA, Baik C, Su WC, et al. Efficacy and Safety of Patritumab Deruxtecan (HER3-DXd) in EGFR Inhibitor-Resistant, EGFR-Mutated Non-Small Cell Lung Cancer. Cancer Discov 2022;12:74-89. [Crossref] [PubMed]
- Shu C A, et al. Amivantamab plus lazertinib in post-osimertinib, post-platinum chemotherapy EGFR-mutant non-small cell lung cancer (NSCLC): Preliminary results from CHRYSALIS-2. Ann Oncol 2021;32:S952-S953. [Crossref]


