
An exploration of the clinical progression models of osimertinib in the treatment of advanced EGFR-mutant non-small cell lung cancer
Introduction
Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) when compared with chemotherapy, in first-line treatment of advanced non-small cell lung cancer (NSCLC) harboring EGFR sensitizing mutations, significantly improve the progression-free survival (PFS) and the objective response rate (ORR) (1-5). However, nearly all patients using first-and second-generation EGFR-TKIs will eventually develop acquired resistance, with the EGFR Exon 20 T790M mutation providing the most common mechanism (6,7). Osimertinib, third-generation EGFR-TKI, showed activity and efficacy against EGFR sensitizing mutations and EGFR T790M mutation of resistance including central nervous system and leptomeningeal metastases (8-10). The FALURA trial showed that osimertinib when compared with the first-generation EGFR-TKIs, gefitinib or erlotinib, in first-line therapy of advanced NSCLC harboring sensitizing EGFR mutations, significantly improve the PFS and overall survival (OS) of patients with advanced NSCLC (11-14). Moreover, the ADAURA clinical trial proved that osimertinib can extend the disease-free survival of patients with stage IB to IIIA EGFR mutation-positive NSCLC after surgery (15).
As one of the most widely used third-generation EGFR-TKIs, osimertinib also faces the widespread clinical problem of acquired resistance. Previous studies have described a series of resistance mechanisms, including EGFR C797 mutation (16), MET amplification (17), mammalian target of rapamycin (MTOR) mutation (18), cluster of differentiation 74 (CD74) upregulation (19), and intercellular transfer of exosomal wild type EGFR (20). The diversity of TKI failure has been analysed sporadically, but a clinical exploration of the failure mode of third-generation EGFR-TKI treatment which can describe and classify the resistance as a whole is still lacking. A research team led by Yilong Wu classified the progression pattern of first-generation EGFR-TKIs in 2012, and subsequent Chinese Society of Clinical Oncology (CSCO) guidelines have also formulated different treatment strategies according to the progression pattern (21,22). As the first third-generation EGFR-TKI that specifically targets EGFR T790M resistance mutations in China market, osimertinib differs significantly from first- and second-generation EGFR-TKIs in terms of resistance mechanisms, and its corresponding progression patterns may also differ. Excepting the Reiwa, which aims to evaluate the progression patterns of first-line osimertinib treatment on Response Evaluation Criteria in Solid Tumors (RECIST)-defined progressive disease (PD) cases and subsequent treatment from September 2018 to August 2022 (23), there has not been any large-scale research that evaluated the progression pattern of osimertinib whether in the first-line treatment or in the second-line treatment. The correlation between the progression pattern of osimertinib and the PFS and OS of patients and the proportion of different progression patterns are not yet known.
Using real-world data, this study aimed to propose a classification system based on the time course of tumor lesion changes and routine clinical examinations in osimertinib treatment of EGFR-mutant advanced NSCLC and to evaluate the role of this classification method in predicting treatment effect and progression after resistance. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-315/rc).
Methods
Study population
A total of 1,125 patients with lung cancer who attended the Affiliated Cancer Hospital of Nanjing Medical University were screened from April 1, 2017 to December 31, 2018, and 117 advanced NSCLC cases with EGFR-positive mutation who had been treated with osimertinib were collected and analyzed. Patients included in the study met the following criteria: (I) over 18 years old; (II) pathologically confirmed as stage IIIB or IV NSCLC; (III) with an Eastern Cooperative Oncology Group (ECOG) score of 0 to 2; (IV) without any serious underlying diseases such as dysfunction of heart, kidney, and liver; (V) with positive EGFR Exon20 T790M mutations.
The clinical characteristics, including gender, age, treatment lines, smoking history, metastases, and the number and site of the metastases outside the primary lesion, were recorded and collected. The number of patients who met the enrollment criteria in this study determined the sample size.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Nanjing Medical University (No. 538), and all participants signed an informed consent form.
Therapeutic method
After enrollment, the patients were given a standard osimertinib regimen orally with a dose of 80 mg once a day before meals or a reduced dose of 40 mg once a day before meals, which was suitable for patients with severe or moderate adverse drug reactions. If patients remained intolerant or progressed when the daily dose was reduced to 40 mg, the drug was permanently discontinued, and this was defined as the endpoint of follow-up.
Efficacy evaluation
The efficacy of osimertinib was evaluated every 4 to 6 weeks according to the 4 categories of RECIST1.1: PD, stable disease (SD), partial response (PR), and complete response (CR). PFS was defined as the time from the patient’s first use of osimertinib to tumor progression or death. The time interval between each efficacy evaluation was adjusted according to clinical reality and some new or anabatic symptoms such as pain or stuffiness. The primary end point was PFS.
Radiological evaluation
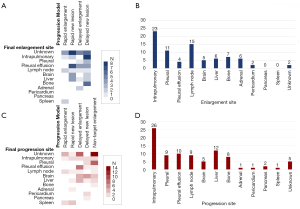
The head, chest, and abdomen of the patients were examined by computed tomography (CT; normal scan + enhanced scan) imaging every 4 to 6 weeks in the radiology department of the Affiliated Cancer Hospital of Nanjing Medical University. To reduce potential bias, all image data were interpreted by at least 2 senior professional radiologists and 2 senior professional oncologists to determine whether the target lesion was enlarged, whether there were new lesions, and whether the patient’s condition had progressed. The patients who experienced PD were divided into a ‘Rapid Type’ and a ‘Delayed Type’ group according to the course of the progression. The ‘Rapid Type’ group included 2 progression models, ‘Rapid Enlargement’ and ‘Rapid New Lesion’, and the ‘Delayed Type’ included 3 progression models, ‘Delayed Enlargement’, ‘Delayed New Lesion’, and ‘Non-target Enlargement’. The definition of each subgroup is shown in detail below (Figure 1).
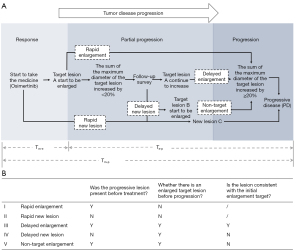
- Rapid enlargement: enlargement of the target lesion is observed on radiological imaging for the first time and meets the standard of PD in RECIST1.1.
- Rapid new lesion: a new target lesion is observed on radiological imaging for the first time.
- Delayed enlargement: the target lesion further increases to meet the standard of PD in RECIST1.1 after initial radiological imaging showed that the target lesion was enlarged but did not meet the standard of PD in RECIST1.1.
- Delayed new lesion: new lesions appear before the target lesion further increases to meet the standard of PD in RECIST1.1 after initial radiological imaging showed that the target lesion was enlarged but did not meet the standard of PD in RECIST1.1.
- Non-target enlargement: a non-target lesion is enlarged and meets the standard of PD in RECIST1.1 before the target lesion further increases to meet the standard of PD in RECIST1.1 after initial radiological imaging showed that the target lesion was enlarged but did not meet the standard of PD in RECIST1.1.
Three time intervals were defined according to the time of the first osimertinib treatment, the time when the target lesion began to enlarge, and the time at which the target lesion met the standard of PD:
- Tmedication-progression(Tm-p): the time interval from the beginning of osimertinib treatment to the development of PD.
- Tmedication-enlargement(Tm-e): the time interval from the beginning of osimertinib treatment to the first observation of target lesion enlargement on imaging. If the enlargement of the target lesion was observed on radiological imaging for the first time and met the standard of PD in RECIST1.1, it was not judged as Tmedication-enlargement(Tm-e) but as Tmedication-progression(Tm-p).
- Tenlargement-progression(Te-p): the time interval from the first observation of target lesion enlargement on imaging to the development of PD.
Statistical analysis
Statistical analysis and visualization of results were performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA), Excel (Windows Excel 365, Microsoft, Redmond, WA, USA), and R 4.1.2 (The R Foundation for Statistical Computing, Vienna, Austria).
The categorical variables in this study were gender, treatment lines, smoking history, metastases, the number and site of the metastases outside the primary lesion, and the classification of progression models, which were expressed as frequencies with constituent ratios and analyzed by chi-square test. The quantitative variables were age, PFS, and the 3 time intervals, which were analyzed and compared by Kaplan-Meier method and log-rank test, t-test, and linear regression. All analyses used a two-tailed P value of <0.05 as the criteria for statistical significance.
Results
The classification of progression models
After screening 1,125 patients with lung cancer from April 1, 2017 to December 31, 2018, among the 265 patients who met the above inclusion criteria, 168 patients had a history of using osimertinib and 51 patients were excluded due to a lack of complete and accurate medical records or loss to follow-up. A total of 117 patients with advanced NSCLC with EGFR-positive mutation who had used osimertinib n the Affiliated Cancer Hospital of Nanjing Medical University were collected and analyzed to evaluate the efficacy of osimertinib (Figure 2). All patients (70 females and 47 males) were in a good general condition with an ECOG score of 0–2. The youngest patient was 29 years old and the oldest was 93 years old with a median age of 60.58. Two patients used osimertinib as a first-line treatment, while 115 patients used osimertinib as a second-line treatment including 50 patients using chemotherapy and 65 patients using 1st/2nd generation EGFR-TKIs as the first-line treat. Twelve patients had a smoking history, and 105 patients had never smoked. The EGFR T790M resistance-related gene mutations of all the 117 patients are positive. In addition, 42.31% of the patients owned positive EGFR Exon19 mutations, while 32.70% of the patients with EGFR Exon21 mutations, 3.85% of the patients with mutations of both EGFR Exon19 and EGFR Exon21. The baseline characteristics of the patients are shown in Table 1.
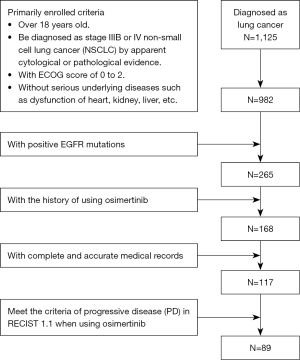
Table 1
| Items | n | % |
|---|---|---|
| Gender | ||
| Male | 47 | 40.17 |
| Female | 70 | 59.83 |
| Age (years) | ||
| <70 | 101 | 86.32 |
| ≥70 | 16 | 13.68 |
| Treatment line | ||
| I | 2 | 1.71 |
| II | 115 | 98.29 |
| The first-line treatment | ||
| Chemotherapy | 50 | 42.74 |
| 1st/2nd generation EGFR-TKI | 65 | 55.56 |
| Smoking history | ||
| Y | 12 | 10.26 |
| N | 105 | 89.74 |
| Metastases | ||
| Y | 102 | 87.18 |
| N | 15 | 12.82 |
| Site of metastases outside the primary lesion | ||
| Intrapulmonary | 92 | 78.63 |
| Pleural | 47 | 40.17 |
| Pleural effusion | 35 | 29.91 |
| Lymph node | 65 | 55.56 |
| Brain | 21 | 17.95 |
| Liver | 15 | 12.82 |
| Bone | 41 | 35.04 |
| Adrenal | 7 | 5.98 |
| Number of metastases outside the primary lesion | ||
| 0 | 15 | 12.82 |
| 1 | 11 | 9.40 |
| 2 | 22 | 18.80 |
| 3 | 28 | 23.93 |
| 4 | 28 | 23.93 |
| 5 | 9 | 7.69 |
| 6 | 3 | 2.56 |
| 7 | 1 | 0.85 |
The median age [min, max]: 62.00 [29, 93]. EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; TKI, tyrosine kinase inhibitor.
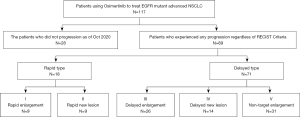
At the final follow-up on October 31, 2020, 28 of the 117 patients were still using osimertinib, and 89 patients stopped treatment due to PD. As shown in Figure 3, 71 of the 89 patients were classified as ‘Delayed Type’, with the target lesion showing partial enlargement on imaging before meeting the standard of PD in RECIST1.1, while the remaining 18 patients were classified as ‘Rapid Type’. In terms of progression models, ‘Non-target Enlargement’ was the most common type, accounting for 31 patients (34.83%), followed by ‘Delayed Enlargement’, accounting for 26 patients (29.21%). ‘Rapid Enlargement’ and ‘Rapid New Lesion’ had the lowest number of patients, accounting for only 9 patients each (10.11%).
Progression after osimertinib resistance
Overall progression process
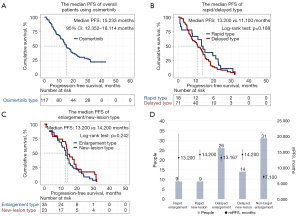
By the end of the study, 89 of the 117 patients stopped osimertinib due to PD, with a longest treatment duration of 43.17 months and an overall median PFS of 15.233 months (95% CI: 12.352–18.114 months; Figure 4A). A total of 71 patients (79.78% of all patients with PD) had partial lesion enlargement on imaging before meeting the standard of PD. The mean Tm-e was 6.59 months after starting osimertinib, and the median Tm-e was 5.47 months. The earliest onset of target lesion enlargement was 0.17 months after using osimertinib and the latest was 24.67 months.
Progression process of different progression models
The median PFS of patients with different progressive models were compared. There was no significant difference between the median PFS of patients with ‘Rapid Type’ and ‘Delayed Type’ progression (13.200 vs. 11.100 months, logrank test; P=0.188; Figure 4B). However, the median PFS of patients with an ‘Enlargement Type’ model (including ‘Delayed Enlargement’, ‘Rapid Enlargement’, and ‘Non-target Enlargement’) and a ‘New Lesion Type’ model (including ‘Rapid New Lesion’ and ‘Delayed New Lesion’) differed but lacked statistically significant (13.200 vs. 14.200 months, logrank test; P=0.242; Figure 4C). The median PFS of patients with different progression models also significantly differed (P=0.046, P<0.05). The median PFS of patients with the progression model ‘Non-target Enlargement’ was the shortest at 7.100 months, while the other progression models had a similar median PFS ranging from 13.167–14.200 months (Table 2 and Figure 4D).
Table 2
| Progression model | n | Ratio | Median PFS (m) | Standard error | 95% CI | P |
|---|---|---|---|---|---|---|
| (I) Rapid enlargement | 9 | 10.11 | 13.200 | 7.553 | (0.000, 28.004) | 0.046 |
| (II) Rapid new lesion | 9 | 10.11 | 14.200 | 1.491 | (11.278, 17.122) | |
| (III) Delayed enlargement | 26 | 29.21 | 13.167 | 1.084 | (11.043, 15.290) | |
| (IV) Delayed new lesion | 14 | 15.73 | 14.200 | 1.653 | (10.961, 17.439) | |
| (V) Non-target enlargement | 31 | 34.83 | 7.100 | 0.634 | (5.858, 8.342) |
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression free survival; CI, confidence interval.
Correlation analysis of Tm-e, Tm-p, and Te-p
Statistical analysis of the Tm-p, Tm-e, and Te-p of patients with the 3 ‘Delayed Type’ progression models (‘Delayed Enlargement’, ‘Delayed New Lesion’, and ‘Non-target Enlargement’), showed that the median and the average Tm-p and Tm-e of patients with ‘Non-target Enlargement’ were significantly shorter than those of patients with ‘Delayed New Lesion’. After the first observation of target lesion enlargement on imaging, there was no significant difference in Te-p among the ‘Delayed Enlargement’, ‘Delayed New Lesion’, and ‘Non-target Enlargement’ models, the averages of which were 5.908, 6.774, and 5.439 months, respectively (Table 3 and Figure 5A-5C).
Table 3
| Items | Delayed enlargement | Delayed new lesion | Non-target enlargement | P | |||||
|---|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | Value | 95% CI | ||||
| Tm-e | |||||||||
| Median | 6.100 | (4.060, 8.140) | 7.333 | (7.273, 7.394) | 3.467 | (2.740, 4.194) | 0.002 | ||
| Average | 8.299 | (5.928, 10.670) | 8.593 | (5.206, 11.980) | 4.246 | (5.357, 7.818) | 0.003 | ||
| Tm-p | |||||||||
| Median | 13.167 | (11.043, 15.290) | 14.200 | (10.961, 17.439) | 7.100 | (5.858,8.342) | 0.041 | ||
| Average | 14.206 | (11.087, 17.326) | 15.367 | (10.263, 20.471) | 9.685 | (7.193, 12.177) | 0.027 | ||
| Te-p | |||||||||
| Median | 3.367 | (0.410, 6.323) | 4.433 | (1.928, 6.939) | 2.267 | (1.661, 2.873) | 0.725 | ||
| Average | 5.908 | (3.842, 7.973) | 6.774 | (2.558, 10.990) | 5.439 | (3.2821, 7.596) | 0.783 | ||
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; CI, confidence interval.

Analyzed by linear regression model in order to determine the quantitative relationship of interdependence between two variables, Tm-e and Tm-p were significantly correlated (P=0.000, P<0.05), with a correlation coefficient of 0.667. Te-p and Tm-p were also significantly correlated (P=0.000, P<0.05) with a correlation coefficient of 0.750. There was no correlation between Tm-e and Te-p (P=0.947, P>0.05). The correlation of the 3 time intervals is shown in Figure 5D. The correlations of Tm-e, Tm-p, and Te-p in the different progression models were all similar to that of the whole, and there was no significant difference in correlation coefficients (Table 4).
Table 4
| Progression model | Tm-e and Tm-p | Tm-e and Te-p | Tm-p and Te-p | |||||
|---|---|---|---|---|---|---|---|---|
| P | Correlation coefficient | P | Correlation coefficient | P | Correlation coefficient | |||
| Rapid enlargement | 0.533 | – | 0.195 | – | 0.500 | – | ||
| Rapid new lesion | 0.000 | 0.958 | 0.136 | – | 0.018 | 0.756 | ||
| Delayed enlargement | 0.000 | 0.749 | 0.838 | – | 0.001 | 0.630 | ||
| Delayed new lesion | 0.039 | 0.557 | 0.961 | – | 0.000 | 0.838 | ||
| Non-target enlargement | 0.000 | 0.657 | 0.194 | – | 0.001 | 0.575 | ||
Post-resistance progression site
A total of 102 patients had metastases outside the primary lesion before starting osimertinib. Intrapulmonary metastases were the most common type of metastasis (92 patients, accounting for 78.63%), followed lymph node metastases (65 patients, accounting for 55.56%), and pleural metastases (47 patients, accounting for 40.17%; Figure 6A). Regarding the number of metastases outside the primary lesion, there were 11 patients with 1 affected area, 12 patients with 2 affected areas, and 28 patients with 3 or 4 affected areas.
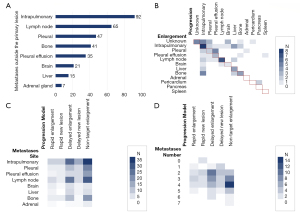
After starting osimertinib, the most common post-resistance progression site was intrapulmonary (29.21%), followed by liver (13.48%), pleural effusion (11.24%), pleural (10.11%), and lymph nodes (10.11%). The average Tm-p of the different clinical progression models differed but was not statistically significant (P=0.093, P>0.05). Adrenal metastasis had the shortest average Tm-p at 7.100 months, with a median Tm-p of 7.100 months. Pericardial metastasis had the longest average Tm-p at 22.700 months, with a median Tm-p of 22.700 months.
Excepting patients with the ‘Rapid Enlargement’ progression type, the earliest enlarged target lesions were intrapulmonary metastases (28.40%), followed by lymph node metastases (18.52%), pleural metastases (13.58%), bone metastases (8.64%), and liver metastases (7.41%). The average Tm-e of different metastatic sites differed but was not statistically significant (P=0.982, P>0.05). As shown in Table 5, pleural effusion tumor cell metastasis had the shortest average Tm-e at 4.858 months, with a median Tm-e of 3.200 months. Brain metastasis had the longest average Tm-e at 8.133 months, with a median Tm-e of 8.800 months.
Table 5
| Site of metastatic lesions | Tm-e | Tm-p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | 95% CI | Average | 95% CI | N | Median | 95% CI | Average | 95% CI | ||
| Intrapulmonary | 19 | 3.933 | (2.180, 5.687) | 6.556 | (3.713, 9.399) | 26 | 9.833 | (5.586, 14.081) | 11.165 | (8.563, 13.767) | |
| Pleural | 10 | 3.800 | (0.000, 8.294) | 5.363 | (1.392, 9.335) | 9 | 13.200 | (0.000, 27.906) | 13.281 | (8.164, 18.399) | |
| Pleural effusion | 4 | 3.200 | (0.946, 5.454) | 4.858 | (0.120, 9.596) | 10 | 10.767 | (4.414, 17.120) | 11.863 | (7.431, 16.296) | |
| Lymph node | 15 | 6.967 | (4.358, 9.576) | 6.822 | (4.602, 9.042) | 9 | 14.133 | (12.283, 15.984) | 16.244 | (10.959, 21.530) | |
| Brain | 4 | 8.800 | (5.664, 11.936) | 8.133 | (6.562, 9.705) | 5 | 9.133 | (2.477, 15.789) | 12.080 | (2.024, 22.136) | |
| Liver | 6 | 4.800 | (1.879, 7.721) | 7.344 | (2.239, 12.450) | 12 | 6.033 | (0.998, 11.069) | 13.622 | (6.960, 20.284) | |
| Bone | 11 | 5.467 | (2.985, 7.948) | 6.952 | (4.250, 9.653) | 8 | 13.200 | (0.000, 28.399) | 10.733 | (5.499, 15.967) | |
| Adrenal | 0 | – | – | – | – | 1 | 7.100 | – | 7.100 | (7.100, 7.100) | |
| Pericardium | 2 | 7.333 | – | 7.333 | – | 1 | 22.700 | – | 22.700 | (22.700, 22.700) | |
| Pancreas | 0 | – | – | – | – | 2 | 18.333 | – | 18.333 | (18.333, 18.333) | |
| Spleen | 0 | – | – | – | – | 1 | 14.200 | – | 14.200 | (14.200, 14.200) | |
| P | 0.983 | 0.982 | 0.783 | 0.800 | |||||||
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; CI, confidence interval.
As shown in Figure 6B, the initial lesion of enlargement was not always the same as the lesion that eventually reached PD. Although intrapulmonary lesion enlargement to PD was the main direction of progression after intrapulmonary lesion enlargement, liver, bone, and pleural metastases after intrapulmonary lesion enlargement were also common. Intrapulmonary metastasis enlargement to PD after the enlargement of bone metastasis was also an important trend of progression.
Characteristics of the progression models
Clinical characteristics
The basic characteristics of the 5 progression types were analyzed (Table 6), and no significant difference was found in the proportions of gender, age, or treatment lines. The proportion of patients with a history of smoking in the ‘Delayed Enlargement’ (11.54%) and ‘Non-target Enlargement’ (25.81%) progression models was higher than that in the other models, and the difference was statistically significant (P=0.046, P<0.05).
Table 6
| Items | Characteristics | Rapid enlargement | Rapid new lesion | Delayed enlargement | Delayed new lesion | Non-target enlargement | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Ratio | N | Ratio | N | Ratio | N | Ratio | N | Ratio | |||||||
| Gender | Male | 5 | 55.56 | 2 | 22.22 | 9 | 34.62 | 8 | 57.14 | 17 | 54.84 | 0.248 | ||||
| Female | 4 | 44.44 | 7 | 77.78 | 17 | 65.38 | 6 | 42.86 | 14 | 45.16 | ||||||
| Age (years) | <70 | 7 | 77.78 | 8 | 88.89 | 21 | 80.77 | 10 | 71.43 | 27 | 87.10 | 0.730 | ||||
| ≥70 | 2 | 22.22 | 1 | 11.11 | 5 | 19.23 | 4 | 28.57 | 4 | 12.90 | ||||||
| Treatment line | I | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | – | ||||
| II | 9 | 100.00 | 9 | 100.00 | 26 | 100.00 | 14 | 100.00 | 31 | 100.00 | ||||||
| The first-line treatment | Chemotherapy | 5 | 55.56 | 2 | 22.22 | 14 | 53.85 | 6 | 42.86 | 20 | 64.52 | 0.224 | ||||
| 1st/2nd generation EGFR-TKI | 4 | 44.44 | 7 | 77.78 | 12 | 46.15 | 8 | 57.14 | 11 | 35.48 | ||||||
| Smoking history | Y | 0 | 0.00 | 0 | 0.00 | 3 | 11.54 | 0 | 0.00 | 8 | 25.81 | 0.046 | ||||
| N | 9 | 100.00 | 9 | 100.00 | 23 | 88.46 | 14 | 100.00 | 23 | 74.19 | ||||||
| Metastases | Y | 0 | 0.00 | 2 | 22.22 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0.048 | ||||
| N | 9 | 100.00 | 7 | 77.78 | 26 | 100.00 | 14 | 100.00 | 31 | 100.00 | ||||||
| Number of metastases outside the primary lesion | 0 | 0 | 0.00 | 2 | 22.22 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0.265 | ||||
| 1 | 1 | 11.11 | 0 | 0.00 | 1 | 3.85 | 3 | 21.43 | 0 | 0.00 | ||||||
| 2 | 2 | 22.22 | 3 | 33.33 | 9 | 34.62 | 4 | 28.57 | 4 | 12.90 | ||||||
| 3 | 3 | 33.33 | 2 | 22.22 | 7 | 26.92 | 3 | 21.43 | 8 | 25.81 | ||||||
| 4 | 2 | 22.22 | 2 | 22.22 | 5 | 19.23 | 3 | 21.43 | 13 | 41.94 | ||||||
| 5 | 1 | 11.11 | 0 | 0.00 | 2 | 7.69 | 1 | 7.14 | 4 | 12.90 | ||||||
| 6 | 0 | 0.00 | 0 | 0.00 | 2 | 7.69 | 0 | 0.00 | 1 | 3.23 | ||||||
| 7 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 0 | 0.00 | 1 | 3.23 | ||||||
| Site of metastases outside the primary lesion | Intrapulmonary | 7 | 77.78 | 7 | 77.78 | 23 | 88.46 | 13 | 92.86 | 30 | 96.77 | 0.929 | ||||
| Pleural | 3 | 33.33 | 3 | 33.33 | 10 | 38.46 | 6 | 42.86 | 19 | 61.29 | ||||||
| Pleural effusion | 2 | 22.22 | 3 | 33.33 | 6 | 23.08 | 6 | 42.86 | 15 | 48.39 | ||||||
| Lymph node | 6 | 66.67 | 3 | 33.33 | 21 | 80.77 | 6 | 42.86 | 25 | 80.65 | ||||||
| Brain | 3 | 33.33 | 1 | 11.11 | 5 | 19.23 | 2 | 14.29 | 4 | 12.90 | ||||||
| Liver | 2 | 22.22 | 0 | 0.00 | 6 | 23.08 | 2 | 14.29 | 5 | 16.13 | ||||||
| Bone | 3 | 33.33 | 3 | 33.33 | 10 | 38.46 | 1 | 7.14 | 16 | 51.61 | ||||||
| Adrenal | 1 | 11.11 | 0 | 0.00 | 1 | 3.85 | 1 | 7.14 | 3 | 9.68 | ||||||
EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
Distribution of lesions
The location and number of metastases outside primary lesions before treatment did not differ significantly among different progression types; however, a higher proportion of lymph node metastases before treatment were observed in patients with an ‘Enlargement Type’ model than in patients with a ‘New Lesion Type’ model (Figure 6C). As shown in Table 6 and Figure 6D, the ‘Non-target Enlargement’ model had the highest proportion of 3 to 4 metastases occurring outside primary lesions before treatment. In the ‘Delayed Enlargement’ model, the number of metastases before treatment was mainly 2 to 3, while those of other progressive models were even lower.
Different progression models also showed significant differences in enlarged lesions (P=0.003, P<0.05) and progressive lesions (P=0.002, P<0.05). Both enlarged and progressive lesions were mainly intrapulmonary metastases. Brain metastasis was more common in patients with the ‘Rapid Enlargement’ model than in those with other models, while patients with the ‘Delayed Enlargement’ model mainly had lung metastasis and lymph node metastasis, followed by liver metastasis and bone metastasis. In patients whose progressive model was ‘Non-target Enlargement’, the enlargement of adrenal (18.75%) and pleural metastases (25.00%) was common, and patients were more likely to discontinue treatment due to increased pleural effusion of exfoliated cells than due to extrapulmonary metastasis (25.81%; Figure 7).
Discussion
This study demonstrated that the failure of third-generation EGFR-TKI treatment can be divided into five models according to the time course of the tumor progression. These models are as follows: (I) rapid enlargement; (II) rapid new lesion; (III) delayed enlargement; (IV) delayed new lesion; and (V) non-target enlargement. Previous studies on the progression pattern of first-generation EGFR-TKIs found that it was reasonable to continue using the current EGFR-TKI combined with systemic chemotherapy even if the disease had partially progressed (21,24-26). Whether a similar principle can be applied to third-generation EGFR-TKIs remains to be explored. In this study, the results showed that the prognosis of patients with a ‘New Lesion Type’ model was better than that of patients with an ‘Enlargement Type’ model. There was no significant difference in prognosis between the ‘Rapid Type’ and ‘Delayed Type’ groups, although the limited sample size and the difficulty in controlling the follow-up time may be the reason for this. What’s more, the patients with missing follow-up data mostly due to rapid progression would have bias to decrease the data of rapid type. Among the 5 progression models, the ‘Non-target Enlargement’ model had the worst prognosis with the shortest duration of disease control, the median PFS of which was only about half that of the other 4 progression models. The difference in time course mainly occurred in the time interval between the start of osimertinib treatment and the time that the lesions first began to enlarge (Tm-e), which was the key means of differentiation between the models. Once the target lesions began to increase, the progression models all followed a similar time course to the PD treatment endpoint, and no significant difference was found. Whether similar progression processes are common in other EGFR-TKI therapies and their underlying mechanisms remains to be confirmed.
The five progression models in this study showed no significant differences in gender, age, or treatment line. In terms of smoking, the patients with a long-term history of smoking were more likely to experience ‘Non-target Enlargement’ or ‘Delayed Enlargement’ progression. In terms of target lesions, the location and number of metastatic lesions outside the primary lesions before treatment had no significant effect on the progression models. It is possible that patients in the ‘Enlargement Type’ models had more lymph node metastases, and that the ‘Non-target Enlargement’ model had more extensive initial areas of metastases, but more statistical evidence is needed to prove the impact of metastatic site, smoking history, and other factors on classification In terms of enlarged and progressive lesions, different progression models showed different tendencies. Brain metastasis was more common in the ‘Rapid Enlargement’ model than in the other 4 models, while the ‘Delayed Enlargement’ model mainly involved lung metastasis and lymph node metastasis. In the ‘Non-target Enlargement’ model, adrenal and pleural metastases appeared to enlarge first, and it was more common for patients to stop treatment due to an increase of pleural effusion caused by exfoliated tumor cells than due to extrapulmonary metastases. In terms of the target lesion itself, different locations of metastases outside the primary lesion led to differences in the site of the first enlarged lesions and the final progressive lesions.
Previous studies have demonstrated that patients with NSCLC with malignant pleural effusion have a higher incidence of positive EGFR mutations, and that EGFR-TKI drugs have a good effect on this type of patient (27,28). The relationship between pleural metastases and malignant pleural effusion was also explored in the present study. For patients who had pleural metastases before treatment, the enlargement of pleural metastases after osimertinib was the main cause of PD. Once malignant pleural effusion started to increase, a constant increase of malignant pleural effusion was common, followed by the enlargement of pleural metastases. An increase of malignant pleural effusion was also relatively common once pleural metastatic lesions had begun to enlarge, and it was more likely than the enlargement of pleural metastases to become the final factor in PD and patient intolerance leading to the termination of drug treatment. Due to its long-term impact on the quality of life, cardiopulmonary function, and nutritional status of patients, the role of increased malignant pleural effusion should not be ignored in the evaluation of PD.
Three time intervals, Tm-e, Te-p, and Tm-p, were used to analyze the data. Statistical analysis showed that either Tm-e or Te-p was correlated with Tm-p, while there was no obvious correlation between Tm-e and Te-p, both of which were independent variables. The progression models did not affect the length of Te-p but had a profound effect on Tm-e and thus on Tm-p, which affected the duration of overall disease control. Tm-e was particularly helpful in predicting the progression model and overall prognosis.
This study had the following limitations. The retrospective research used convenience sampling rather than random sampling. The follow-up time was short and limited by the difficulty of following up an oral drug, and OS was not included in the research analysis. Moreover, the chosen samples were concentrated in Nanjing, and the narrow population sample may have affected the accuracy of the results.
In conclusion, we tried to create a classification system for the failure of third-generation EGFR-TKI drug resistance treatment that consisted of five progression models and two tumor progression stages based on the time course of the tumor progression. The use of these models and stages could be of a bit benefit to the prediction of overall prognosis and could be potential to assist the selection of subsequent treatment strategies. Further research on the potential molecular mechanisms of drug resistance is necessary to clarify the mechanism of the failure of third-generation EGFR-TKIs in patients with EGFR-mutant advanced NSCLC. In addition, in order to have more robust results, more studies to enlarge the sample size, to increase additional study centers, and to evaluate the progression to Osimertinib when given in first-line setting are also of great significance.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This work was supported by National Natural Science Foundation of China (No. 81802277), Jiangsu Institute of Cancer Research (No. ZM2011814), and Jiangsu Youth Fund (No. BK 20181091).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-315/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-315/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-315/coif). KI reports personal fees from Boehringer Ingelheim, AstraZeneca, Pfizer, Eli Lilly, Chugai Pharmaceutical, Merk Sharp & Dohme (MSD), Ono Pharmaceutical, Takeda Pharmaceutical and Taiho Pharmaceutical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Nanjing Medical University (No. 538), and all participants signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Cao M, Chen W. Epidemiology of lung cancer in China. Thorac Cancer 2019;10:3-7. [Crossref] [PubMed]
- Heuckmann JM, Rauh D, Thomas RK. Epidermal growth factor receptor (EGFR) signaling and covalent EGFR inhibition in lung cancer. J Clin Oncol 2012;30:3417-20. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-iv237. Erratum in: Ann Oncol 2019;30:863-70. [Crossref]
- Hanna NH, Robinson AG, Temin S, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol 2021;39:1040-91. [Crossref] [PubMed]
- Engel J, Richters A, Getlik M, et al. Targeting Drug Resistance in EGFR with Covalent Inhibitors: A Structure-Based Design Approach. J Med Chem 2015;58:6844-63. [Crossref] [PubMed]
- Cortot AB, Jänne PA. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev 2014;23:356-66. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Lee J, Choi Y, Han J, et al. Osimertinib Improves Overall Survival in Patients With EGFR-Mutated NSCLC With Leptomeningeal Metastases Regardless of T790M Mutational Status. J Thorac Oncol 2020;15:1758-66. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; Epub ahead of print. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Lin CC, Shih JY, Yu CJ, et al. Outcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic study. Lancet Respir Med 2018;6:107-16. [Crossref] [PubMed]
- Cho BC, Chewaskulyong B, Lee KH, et al. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J Thorac Oncol 2019;14:99-106. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 2015;21:560-2. [Crossref] [PubMed]
- Ou SI, Agarwal N, Ali SM. High MET amplification level as a resistance mechanism to osimertinib (AZD9291) in a patient that symptomatically responded to crizotinib treatment post-osimertinib progression. Lung Cancer 2016;98:59-61. [Crossref] [PubMed]
- Park HR, Kim TM, Lee Y, et al. Acquired Resistance to Third-Generation EGFR Tyrosine Kinase Inhibitors in Patients With De Novo EGFRT790M-Mutant NSCLC. J Thorac Oncol 2021;16:1859-71. [Crossref] [PubMed]
- Kashima Y, Shibahara D, Suzuki A, et al. Single-Cell Analyses Reveal Diverse Mechanisms of Resistance to EGFR Tyrosine Kinase Inhibitors in Lung Cancer. Cancer Res 2021;81:4835-48. [Crossref] [PubMed]
- Wu S, Luo M, To KKW, et al. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol Cancer 2021;20:17. [Crossref] [PubMed]
- Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33-9. [Crossref] [PubMed]
- Zhang Y, Chen G, Chen X, et al. The comparison of EGFR-TKI failure modes and subsequent management between exon 19 deletion and exon 21 L858R mutation in advanced non-small-cell lung cancer. J Cancer 2017;8:1865-71. [Crossref] [PubMed]
- Watanabe K, Yoh K, Hosomi Y, et al. Efficacy and safety of first-line osimertinib treatment and postprogression patterns of care in patients with epidermal growth factor receptor activating mutation-positive advanced non-small cell lung cancer (Reiwa study): study protocol of a multicentre, real-world observational study. BMJ Open 2022;12:e046451. [Crossref] [PubMed]
- Lee YJ, Choi HJ, Kim SK, et al. Frequent central nervous system failure after clinical benefit with epidermal growth factor receptor tyrosine kinase inhibitors in Korean patients with nonsmall-cell lung cancer. Cancer 2010;116:1336-43. [Crossref] [PubMed]
- Kirsch DG, Ledezma CJ, Mathews CS, et al. Survival after brain metastases from breast cancer in the trastuzumab era. J Clin Oncol 2005;23:2114-6; author reply 2116-7. [Crossref] [PubMed]
- Metro G, Sperduti I, Russillo M, et al. Clinical utility of continuing trastuzumab beyond brain progression in HER-2 positive metastatic breast cancer. Oncologist 2007;12:1467-9; author reply 1469-71. [Crossref] [PubMed]
- Yang JC, Sequist LV, Geater SL, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: a combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 2015;16:830-8. [Crossref] [PubMed]
- Zhou BO, Nie J, Yang W, et al. Effect of hydrothorax EGFR gene mutation and EGFR-TKI targeted therapy on advanced non-small cell lung cancer patients. Oncol Lett 2016;11:1413-7. [Crossref] [PubMed]


