
Efficacy and safety of apatinib as second or later-line therapy in extensive-stage small cell lung cancer: a prospective, exploratory, single-arm, multi-center clinical trial
Introduction
Lung cancer is the most common malignancy worldwide, with more than 787,000 new cases diagnosed and 631,000 deaths in China in 2015 (1). Small cell lung cancer (SCLC) accounts for approximately 15–20% of total lung cancer cases (2), of which more than two thirds are extensive stage (ES) at the time of diagnosis (3). As a recalcitrant malignancy, the 2-year survival rate of ES-SCLC is less than 5% (4) attributable to the elusive pathophysiology, aggressive biology, and stagnated therapeutic progress of ES-SCLC in the past decades. The combination of platinum and etoposide was the cornerstone of therapy for first-line SCLC (5), until the Phase III IMpower133 (6) and CASPIAN (7) trials demonstrated remarkable superiority of chemoimmunotherapy in extending overall survival (OS) by adding programmed cell death protein 1 (PD-1) axis inhibitors to platinum-based chemotherapy. Based on these pivotal Phase III data, the American Food and Drug Administration (FDA) and the National Medicine Products Administration (NMPA) of China approved the combination of carboplatin, etoposide, and the anti-programmed cell death ligand 1 (PD-L1) antibody atezolizumab as a first-line treatment, which was a pivotal advance for SCLC treatment.
Despite the fact that SCLC is sensitive to initial therapy, up to 80% of patients will experience relapse and eventually die from systemic metastases (8). However, until 2020, topotecan was the only recommended second-line therapy with an objective response rate (ORR) not exceeding 25% and OS of 6–9 months (9). It held this status based on its ability to prolong OS compared with best supportive care (10) and noninferiority in controlling symptom versus cyclophosphamide, doxorubicin, and vincristine in patients relapsing from front-line chemotherapy (11). Therefore, it is urgently necessary to develop more effective therapeutic strategies for chemotherapy-refractory SCLC.
Emerging evidence has demonstrated that angiogenesis is a vital facilitator during the growth, invasion, and metastasis of SCLC, during which vascular endothelial growth factor (VEGF) is one of the most important mediators of tumor angiogenesis, whose high-level expression was also shown to be associated with inferior prognosis for SCLC (12,13). Therefore, the anti-VEGF/vascular endothelial growth factor receptors-2 (VEGFR-2) axis is an attractive target for the treatment of SCLC, which underlines a convergence with non-small-cell lung cancer (NSCLC). Furthermore, this concept has been verified in several anti-angiogenic clinical trials of ES-SCLC (14). However, the clinical trials of bevacizumab (an anti-VEGF antibody) and sunitinib (a multi-targeted receptor tyrosine kinase inhibitor) in previously untreated SCLC patients demonstrated that the results do not meet expectations, despite some improved progression-free survival (PFS) and/or OS (15-17). It follows then that the magnitude of benefit from anti-angiogenic therapy in SCLC patients is limited. Therefore, additional studies of novel anti-angiogenesis agents and strategies for ES-SCLC are warranted.
Apatinib is an orally administered small molecular tyrosine kinase inhibitor (TKI) that selectively inhibits VEGFR-2 (18). It has been approved for the third-line or beyond treatment of advanced gastric cancer in China (19) and the second-line or later-line treatment of hepatocellular carcinoma (20). Furthermore, apatinib has shown efficacy in other solid tumors with a high level of safety, such as in NSCLC (21), breast cancer (22), gestational trophoblastic neoplasia (23), cervical cancer (24), esophageal squamous cell carcinoma (25), chordoma (26), and osteosarcoma (27), in monotherapy or combined therapy.
Apatinib has been demonstrated to exert an active anti-tumor effect on SCLC, not only in vitro and in vivo research (28), but also in several clinical trials in ES-SCLC (29-32). When used alone, apatinib achieved an ORR of 17.5% in per-protocol population with a median PFS of 3.0 months and a median OS of 5.8 months in third-line or beyond treatment of ES-SCLC (29). Meanwhile, apatinib monotherapy was well tolerated with manageable toxicity, as that the incidence of the most common grade 3 or greater adverse events (AEs) were hypertension (25%), hand-foot syndrome (10%), L-gamma glutamyltransferase increased (10%), aspartate aminotransferase increased (7.5%), and thrombocytopenia (7.5%). Besides, dose reduction occurred in 37.5% of patients in safety analysis during treatment (29). Additionally, long-term efficacy of adding low-dose apatinib, during the chemotherapy interval and maintenance therapy following chemotherapy in the first-line treatment of ES-SCLC, increased median PFS by 2.9 months (7.8 vs. 4.9 months) and median OS by 3.9 months (12.1 vs. 8.2 months) (31). When apatinib was combined with camrelizumab, an anti-PD-1 antibody, the confirmed ORR reached 34.0% with the median PFS and OS of 3.6 and 8.4 months, respectively, in patients who had progressed on platinum-based first-line chemotherapy (30). Together, though apatinib has been demonstrated to have promising anti-tumor activity with a good safety profile in third-line or beyond treatment of ES-SCLC, it is not clear about the efficacy and safety of apatinib monotherapy in ES-SCLC patients with progression after first-line chemotherapy. Thus, we designed this prospective study to evaluate the efficacy and safety of apatinib in the second- or third-line treatment of ES-SCLC patients. We present the following article in accordance with the TREND reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-313/rc).
Methods
Study design and patients
This is a prospective, exploratory, open-label, multicenter, single-arm clinical trial. Eligible patients were enrolled at 16 hospitals or medical centers in China. To be enrolled, patients had to be older than 18 years; have histologically diagnosed ES-SCLC according to the Veterans Administration Lung Cancer Study Group (VALSG) staging system; failed at least 1 line of chemotherapy; at least 1 measurable lesion as defined by Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1); Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 2; estimated survival duration of ≥3 months; and adequate renal (creatinine ≤120 mol/L) and hematologic functions.
Exclusion criteria included: (I) participation in other research in the prior 4 weeks; (II) dysphagia, chronic diarrhea, or intestinal obstruction; (III) central nervous system metastases; (IV) uncontrolled hypertension (i.e., systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, despite optimal drug treatment); (V) history of hemorrhage or severe cardiovascular disease, such as unstable angina, myocardial infarction within 6 months before enrollment, poorly controlled arrhythmias (including men with QTc interval ≥450 ms, women ≥470 ms) according to New York Heart Association criteria, grades III to IV insufficient function; (VI) urinary protein ≥++; (VII) severe traumatic injury or fracture; (VIII) abnormal blood coagulation or a clear tendency to hemorrhage, low-dosage warfarin or aspirin were allowed as prevention if international normalized ratio (INR) ≤1.5; (IX) events of arterial and/or venous thrombosis occurring within 6 months before enrollment such as cerebrovascular accidents (including transient ischemic attacks), deep vein thrombosis, and pulmonary embolism; (X) pregnancy; and (XI) history of thyroid dysfunction, psychoactive substance abuse, immunodeficiency, hydrothorax, or ascites.
This study is registered with the China Trial Register, number ChiCTR-OPC-17013964. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol and amendments were reviewed and approved by the Ethics Review Committee of The Fourth People’s Hospital of Wuxi (which is the former name of Affiliated Hospital of Jiangnan University) (No. LS2017045). All participating institutes were informed and agreed the study. All patients provided written informed consent before study entry.
Treatment
Patients received apatinib (500 mg orally qd) 30 min after meals, continuously in 4-week cycles. Apatinib was taken at home until disease progression, intolerable toxicities, physician or patient’s decision, or death. If severe AEs occurred, the dosage could be reduced to 250 mg. If participants could not tolerate apatinib at dosage of 250 mg qd, they were recommended to take apatinib on an intermittent schedule with 3 weeks on/1 week off. Treatment interruption resulting from toxicities was allowed for no more than 14 days. If patients still could not tolerate 250 mg, they were removed from the study. All participants took vitamin B6 3 times daily in order to prevent hand-foot syndrome, a common AE of apatinib. Nonsteroidal anti-inflammatory drugs and dexamethasone were used to treat hand-foot syndrome.
Assessment and endpoints
Tumor assessments were performed by radiographic imaging, including computed tomography (CT) and magnetic resonance imaging (MRI), at baseline, after 1 cycle of apatinib treatment, and then every 2 cycles (±7 days) thereafter until disease progression. During follow-up, the CT imaging of the chest and abdomen were conducted, and enhanced MRI if necessary. Laboratory biochemical tests, such as serum chemistry, routine urine examinations, and vital signs, such as physical examinations and 12 lead electrocardiograms, were monitored every month during treatment. Survival was followed up continuously during treatment, and every 2 months after discontinuation of apatinib.
The efficacy analysis was conducted in the full analysis set (FAS) including participants who received at least 1 dose of apatinib without serious protocol violation. The primary endpoint was PFS, which was defined as the time from the date of entry to that of disease progression or death from any cause. Secondary endpoints included OS, ORR, disease control rate (DCR), and safety. The OS was defined as the time from entry to the date of death. The ORR included the complete response (CR) and partial response (PR); the DCR included the CR, PR, and stable disease (SD). Tumor response and progression were assessed according to RECIST 1.1 (33,34). The safety analysis was conducted among participants who received at least 1 dose of apatinib, except those without any safety data. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE 4.0; https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf).
Statistical analysis
A total of 60 eligible ES-SCLC patients was designed to enroll in this exploratory study. The demographic data, outcome data, and other clinical parameters were presented as the frequency for categorical variables, and the median with interquartile range (IQR) for age variable. For ORR and DCR, point estimates and exact Clopper-Pearson confidence intervals (CIs) were provided. Median PFS and OS were estimated by Kaplan-Meier methodology and were reported with 95% CIs. Log-rank tests were used for survival endpoints among subgroups. Cox proportional hazards models were used for multivariate analysis to estimate the prognostic factors for PFS and OS. The prognostic factors were identified statistical significance if P<0.05. All the statistical data were analyzed using SAS 9.4 (SAS Institute, Cary, NC, USA) with two-sided testing (α=0.05). A P value <0.05 was considered significant.
Results
Participant characteristics
From 28 July 2017 to 21 June 2019, 62 patients were screened, of whom 57 eligible patients received apatinib monotherapy as second-line or beyond treatment and included in the FAS (Figure 1). The baseline characteristics of the participants are shown in Table 1. Their median age was 61 years (IQR, 55–66 years), and 46/57 (80.70%) were male. Primary tumors were in the right lung in 68.4% (39/57) with 31.6% (18/57) in the left. A total of 18 (31.6%) patients had ≥3 metastatic lesions and 7 (12.3%) participants did not have a metastatic lesion. More than half (52.6%, 30/57) of the patients received apatinib as second-line treatment, while 42.1% (24/57) and 5.3% (3/57) received it as third- and fourth-line treatment, respectively. The prior chemotherapy regimens or prior anticancer therapies are summarized in Table S1. Briefly, etoposide and platinum in first-line treatment and irinotecan based or in combination with platinum in the second-line were the most common regimens, in 77.2% and 66.7% patients, respectively.
Table 1
| Characteristic | ES-SCLC participants (N=57) | Apatinib Treatment Line | ||
|---|---|---|---|---|
| Second-line (n=30) | Third-line (n=24) | ≥ Forth-line (n=3) | ||
| Age, years, median [IQR] | 61 [55–66] | 61 [57–66] | 62 [53–66] | 66 [65–73] |
| Age, n (%) | ||||
| <60 years | 19 (33.3) | 8 (26.7) | 11 (45.8) | 0 (0.0) |
| ≥60 years | 38 (66.7) | 22 (73.3) | 13 (54.2) | 3 (100.0) |
| Gender, n (%) | ||||
| Male | 46 (80.7) | 22 (73.3) | 22 (91.7) | 2 (66.7) |
| Female | 11 (19.3) | 8 (26.7) | 2 (8.3) | 1 (33.3) |
| ECOG score, n (%) | ||||
| <2 | 31 (54.4) | 15 (50.0) | 15 (62.5) | 1 (33.3) |
| 2 | 26 (45.6) | 15 (50.0) | 9 (37.5) | 2 (66.7) |
| Primary lesion, n (%) | ||||
| Right lung | 39 (68.4) | 23 (76.7) | 14 (58.3) | 2 (66.7) |
| Left lung | 18 (31.6) | 7 (23.3) | 10 (41.7) | 1 (33.3) |
| Apatinib treatment Settings, n (%) | – | 30 (52.6) | 24 (42.1) | 3 (5.3) |
| Prior surgical resection, n (%) | ||||
| Yes | 1 (1.75) | 0 (0.0) | 1 (4.2) | 0 (0.0) |
| No | 54 (94.7) | 29 (96.7) | 22 (91.7) | 3 (100.0) |
| Missing | 2 (3.5) | 1 (3.3) | 1 (4.2) | 0 (0.0) |
| Radiotherapy, n (%) | ||||
| Yes | 45 (79.0) | 23 (76.7) | 19 (79.2) | 3 (100.0) |
| No | 10 (17.1) | 6 (20.0) | 4 (16.7) | 0 (0.0) |
| Missing | 2 (3.5) | 1 (3.3) | 1 (4.2) | 0 (0.0) |
| Metastatic lesion number, n (%) | ||||
| 0 | 7 (12.3) | 4 (13.3) | 3 (12.5) | 0 (0.0) |
| 1 | 18 (31.6) | 10 (33.3) | 6 (25.0) | 2 (66.7) |
| 2 | 14 (24.6) | 8 (26.7) | 5 (20.8) | 1 (33.3) |
| ≥3 | 18 (31.6) | 8 (26.7) | 10 (41.7) | 0 (0.0) |
ES-SCLC, extensive stage small cell lung cancer; IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group.
Efficacy
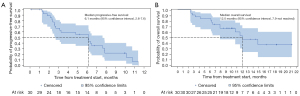
With a median follow-up of 8.6 months (range, 1.0–24.3 months), 49 of 57 patients were eligible for efficacy analysis in FAS. The other 8 patients were not evaluable for efficacy analysis, with 3 patients withdrawing informed consent and 5 patients discontinuing treatment for AEs before efficacy evaluation (4 hypertension and 1 neutrophil count decreased combined with concomitant white blood cell decreased). According to the investigator assessment, PR was achieved in 7 (14.3%) cases, and SD was exhibited in 32 (65.3%) cases, with no CR (Table 2). A total of 10 (20.4%) participants experienced progressive disease (PD) with 3 deaths occurring in the first cycle. The ORR and DCR were 14.3% and 79.6% in this study, respectively. In addition, the median PFS was 5.6 months (95% CI: 3.4–6.5 months) (Figure 2A), while the median OS was 11.2 months (95% CI: 7.5–24.0 months) (Figure 2B).
Table 2
| Clinical outcome | ES-SCLC patients (N=49) | Apatinib treatment line | ||
|---|---|---|---|---|
| Second-line (n=24) | Third-line (n=22) | ≥ Forth-line (n=3) | ||
| CR, n (%) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.0) |
| PR, n (%) | 7 (14.3) | 3 (12.5) | 4 (18.2) | 0 (0.0) |
| SD, n (%) | 32 (65.3) | 18 (75.0) | 11 (50.0) | 3 (100.0) |
| PD, n (%) | 10 (20.4) | 3 (12.5) | 7 (31.8) | 0 (0.0) |
| ORR (%), median (95% CI) | 14.3 (4.5–24.1) | 12.5 (0.0–25.7) | 18.2 (2.01–34.3) | 0 (0.0) |
| DCR (%), median (95% CI) | 79.6 (68.3–90.9) | 87.5 (74.3–100) | 68.2 (48.7–87.6) | 100.0 |
| PFS (months), median (95% CI) | 5.6 (3.4–6.5) | 6.1 (2.6–7.6) | 5.4 (1.8–6.5) | 3.6 (3.2–12.0) |
| OS (months), median (95% CI) | 11.2 (7.5–24.0) | 12.0 (7.9–NR) | 7.5 (5.6–24.0) | 11.2 (7.1–14.1) |
ES-SCLC, extensive stage small cell lung cancer; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; NR, not reached; CI, confidence interval.

Among the participants who received apatinib as second- and third-line treatment, 24 out of 30 and 22 out of 24 cases were evaluable for efficacy analysis, respectively. In the second-line treatment, the ORR and DCR were 12.5% and 87.5%, respectively, resulting from 3/24 (12.5%) PR, 18/24 (75.0%) SD, and 3/24 (12.5%) PD; meanwhile, the median PFS and OS were 6.1 months (95% CI: 2.6–7.6 months) and 12.0 months (95% CI: 7.9 months to not reached), respectively (Figure 3). Furthermore, in the third-line therapy, the ORR and DCR were 18.2% and 68.2%, respectively, with 4/22 (18.2%) PR, 11/22 (50.0%) SD, and 7/22 (31.8%) PD, and the median PFS and OS of patients who received apatinib as third-line treatment were 5.4 months (95% CI: 1.8–6.5 months) and 7.5 months (95% CI: 5.6–24.0 months), respectively (Figure 4).

Multivariate analysis
According to Cox multivariate regression analysis, the association between the clinical variables and survival outcomes, ECOG PS of 2 is significantly correlated with PFS [P=0.01, hazard ratio (HR) =4.26]. The occurrence of hand-foot syndrome (P=0.01, HR =0.09), fatigue (P=0.01, HR =6.37) and oral mucositis (P=0.01, HR =43.87) was significantly associated with longer PFS (Table 3). All the variables were not found to have significant effect on OS.
Table 3
| Factors | PFS | OS | |||
|---|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR (95% CI) | ||
| Age group (≥60 years) | 0.62 | 1.36 (0.4–4.63) | 0.53 | 1.68 (0.33–8.58) | |
| Gender (male) | 0.44 | 1.82 (0.4–8.31) | 0.45 | 2.17 (0.29–16.06) | |
| ECOG PS (=2) | 0.01 | 4.26 (1.41–12.92) | 0.64 | 0.75 (0.23–2.47) | |
| Hypertension as AE | 0.46 | 0.59 (0.14–2.40) | 0.23 | 2.8 (0.52–15.18) | |
| Proteinuria as AE | 0.30 | 2.43 (0.45–13.04) | 0.61 | 0.44 (0.02–10.24) | |
| Hand-foot syndrome as AE | 0.01 | 0.09 (0.01–0.62) | 0.16 | 0.19 (0.02–1.89) | |
| Mucositis oral as AE | 0.01 | 43.87 (2.22–866.43) | 0.77 | 1.66 (0.05–51.25) | |
| Fatigue as AE | 0.01 | 6.37 (1.71–23.71) | 0.83 | 1.18 (0.27–5.12) | |
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; AE, adverse event.
AEs
All 57 participants were included in the safety analysis. The most common AEs were anemia (36.8%), hypertension (33.3%), fatigue (31.6%), blood bilirubin increased (22.8%), elevated transaminase (19.3%), and hand-foot syndrome (17.5%). Most of the AEs were grade 1–2, which were alleviated after reducing apatinib dose or symptomatic treatment. Grade 3 AEs only occurred in 3 participants, 1 case for each of hypertension, fatigue, or headache (Table 4). No grade 5 AE was observed. Six patients discontinued apatinib treatment for hypertension (1 case of grade 1, 2 cases of grade 2, and 1 case of grade 3), neutrophil count decreased combined with white blood cell decreased (1 case of grade 2), and fatigue (1 case of grade 2).
Table 4
| Events | Grade 1 | Grade 2 | Grade 3 | Total |
|---|---|---|---|---|
| Anemia | 21 (36.8) | 0 | 0 | 21 (36.8) |
| Blood bilirubin increased | 11 (19.3) | 2 (3.5) | 0 | 13 (22.8) |
| Elevated transaminase | 10 (17.5) | 1 (1.7) | 0 | 11 (19.3) |
| Neutrophil count decreased | 9 (15.8) | 1 (1.7) | 0 | 10 (17.5) |
| Platelet count decreased | 8 (14.0) | 1 (1.7) | 0 | 9 (15.8) |
| Alkaline phosphatase increased | 4 (7.0) | 3 (5.3) | 0 | 7 (12.3) |
| Creatinine increased | 4 (7.0) | 0 | 0 | 4 (7.0) |
| Hypertension | 10 (17.5) | 7 (12.3) | 2 (3.5) | 19 (33.3) |
| Fatigue | 14 (24.6) | 3 (5.3) | 1 (1.8) | 18 (31.6) |
| Hand-foot syndrome | 3 (5.3) | 7 (12.3) | 0 | 10 (17.5) |
| Headache | 9 (15.8) | 0 | 1 (1.8) | 10 (17.5) |
| Rash maculo-papular | 2 (3.5) | 1 (1.8) | 0 | 3 (5.3) |
| Mucositis oral | 2 (3.5) | 1 (1.8) | 0 | 3 (5.3) |
| Proteinuria | 2 (3.5) | 4 (7.0) | 0 | 6 (10.5) |
| Diarrhea | 2 (3.5) | 3 (5.3) | 0 | 5 (8.8) |
| Rash | 2 (3.5) | 1 (1.8) | 0 | 3 (5.3) |
| Cough | 2 (3.5) | 1 (1.8) | 0 | 3 (5.3) |
| Nausea | 1 (1.8) | 1 (1.8) | 0 | 2 (3.5) |
| Hoarseness | 1 (1.8) | 0 | 0 | 1 (1.8) |
Data are n (%). ES-SCLC, extensive stage small cell lung cancer.
Discussion
To our knowledge, this is the first registered and the largest clinical trial to investigate the feasibility of apatinib monotherapy in second-line or beyond treatment of ES-SCLC. This study met all its primary and secondary endpoints. The results indicated that apatinib monotherapy provides encouraging efficacy and tolerable toxicity for ES-SCLC patients who fail first-line regimens. For evaluable patients, the ORR and DCR were 14.3% and 79.6%; the median PFS and OS were 5.6 and 11.2 months, respectively. In the subgroup analysis, second-line treatment of apatinib demonstrated an ORR of 12.5% and a DCR of 87.5% and favorable survival result with a median PFS of 6.1 months and a median OS of 12.0 months. Furthermore, apatinib demonstrated potential efficacy by achieving the ORR and DCR to 18.2% and 68.2%, respectively, and demonstrating median PFS and OS of 5.4 and 7.5 months, respectively, in third-line treatment ES-SCLC.
Currently, topotecan remains the only chemotherapeutic agent approved by the FDA for second-line treatment of SCLC in the last 2 decades (10,35). Treatment of topotecan yielded a response rate of 10–40% and a median survival of 6.0–8.2 months after failure with etoposide and platinum-based combination regimen (10,35,36). Bevacizumab, another antiangiogenic agent, was also investigated in ES-SCLC for second-line treatment, demonstrating a median PFS of 2.7–4 months and a median OS of 6.3–7.4 months (37). In 2020, lurbinectedin emerged as a promising cytotoxic agent which exhibited promising efficacy in second-line treatment of ES-SCLC with an ORR of 35.2%, a DCR of 68.6%, and favorable survival with a median PFS of 3.5 months and a median OS of 9.3 months in a single-arm, phase 2 basket trial (38). In our study, apatinib demonstrated a survival benefit of up to 12.0 months accompanied by a very encouraging DCR of 87.5%. Acknowledging the inescapable limitations of cross-study comparisons, apatinib showed a dominant DCR and survival benefit in second-line treatment of ES-SCLC when compared with topotecan, bevacizumab, and lurbinectedin. These results indicate that apatinib might be a promising agent for second-line treatment of ES-SCLC.
There have been two other prospective studies exploring the safety and efficacy of apatinib in third-line or beyond treatment of ES-SCLC (29,32). Apatinib could achieve an objective response in 13.6–18.4% and disease control in 78.9–95.5% of participants, and prolong the median PFS and OS to the extent of 3.0–5.4 months and 5.8–10.0 months, respectively (29,32). In this study as third-line treatment, the efficacy of apatinib in ORR and DCR were aligned with those in previous studies, or potentially had even better PFS and OS although comparisons across studies are difficult to interpret (29,32). Moreover, anlotinib, an orally administered TKI, approved for third-line treatment of ES-SCLC by NMPA, significantly prolonged PFS when compared with placebo (4.1 vs. 0.7 months, P<0.0001) in third-line treatment of SCLC in the ALTER 1202 study. Other investigations involving inhibitors of PD-1/PD-L1 axis have been conducted in patients diagnosed with SCLC. The KEYNOTE-158 trial showed that the PFS was 2.0 months among SCLC patients who received pembrolizumab as third- or later-line treatment (39); CheckMate-032 demonstrated a PFS of 1.4 months among SCLC patients who received nivolumab monotherapy after failing from 2 or more previous chemotherapy regimens (40). Though monotherapy of nivolumab and pembrolizumab gained the accelerated approval of FDA as third-line treatments for SCLC, they were withdrawn considering they did not meet the primary endpoints in confirmatory phase III studies. All the above studies demonstrated shorter median PFS and OS than the present study (Figure S1).
Apatinib has been demonstrated to have direct anti-tumor effect and anti-angiogenic effect in pre-clinical studies. The mechanisms of direct anti-tumor effect of apatinib may involve the inhibition of activating AKT and ERK1/2, and enhancing anti-tumor effect by inducing production of 3-hydroxybutyric acid, which is a class I histone deacetylase inhibitor, via activating proliferator-activated receptor α (41-43). In the setting of anti-angiogenesis, apatinib selectively inhibits the kinase activity VEGFR-2, which play pivotal roles in the migration of endothelial cell and tube formation, truncating the blood supply in intratumor environment (18). It is noteworthy that the ORR of apatinib in the third-line treatment is numerically higher than those in the second-line. The reason may be partly concerned with the more extensive angiogenesis in patients with third-line treatment, who responded better to anti-angiogenesis of apatinib. Additionally, the better response to apatinib in later-line ES-SCLC is in line with the effect of apatinib in later-line hepatocellular carcinoma, who were refractory to the combination treatment of sorafenib and transarterial chemoembolization (44). Furthermore, the survival benefits observed in the present study appear also comparable to the standard first-line chemotherapy regimen with or without immunotherapy (6,45). However, these data should be validated in a phase III study with adequate power and a control group. Although direct comparisons across studies with different designs and patient populations are not ideal, our study indicates that active systemic therapy in pre-treated ES-SCLC patients is clinically valuable.
The most common AEs reported by previous study of the apatinib monotherapy in patients with SCLC who failed two or more lines treatment included secondary hypertension (57%), proteinuria (48%), oral mucositis (29%), and hand-foot syndrome (19%) (32). Our study demonstrated similar acceptable safety profile with only mild to moderate severity of toxicity. Hypertension and hand-foot syndrome were 2 of the most common treatment-related AEs, with incidence rates of 33.33% and 17.54%, respectively, which could be managed by corresponding medications. Hand-foot syndrome is one of the common AEs in patients treated with apatinib (32,46). VEGFR has been reported as primarily responsible for this side effect (47,48). It has been reported that the clinical benefit was significantly improved in advanced HCC patients who developed VEGF inhibitor induced hand-foot syndrome compared with those that did not (49). Consistently, multivariate analysis from our study revealed treatment-associated hand-foot syndrome as an independent predictor of PFS which indicated the occurrence of hand-foot syndrome in response to apatinib could potentially serve as a predictive biomarker for ES-SCLC treatment. However, further studies with a large sample size are necessary to confirm.
Conclusions
Overall, based on the efficacy and toxicity profile, apatinib displayed clear efficacy and manageable toxicity in ES-SCLC patients who failed first-line chemotherapy. Apatinib monotherapy might be a reasonable option to the challenge of advanced SCLC treatment after failure of initial chemotherapy. As the present study was an exploratory single-arm clinical trial, our conclusion is severely limited by the small sample and lack of a control group for comparison. A randomized multicenter clinical trial with a larger cohort is necessary to confirm our findings.
Acknowledgments
The authors would like to extend their gratitude to all the patients who participated in this study and their families, the investigators, study coordinators, operation staff, and the whole project team who worked on this study. The authors would also like to acknowledge Yaqi Li (a Medical Writer employed by Jiangsu Hengrui Pharmaceuticals Co., Ltd.) for medical writing support according to Good Publication Practice Guidelines. The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This study was supported by the Precision Medicine Project of Wuxi Health Commission (No. jzyx04), Translational Medicine Research Project of Wuxi Health Commission (No. ZH202103), and the Clinical Research Center Fund from Wuxi Science and Technology Bureau (No. WX18IVJN017).
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-313/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-313/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-313/coif). SKJ reports that she receives institutional grant from Merck & Co., Inc. and consulting fees form Merck & Co., Inc., Syntactx and IMX Medical. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol and amendments were reviewed and approved by the Ethics Review Committee of The Fourth People’s Hospital of Wuxi (which is the former name of Affiliated Hospital of Jiangnan University) (No. LS2017045). All participating institutes were informed and agreed the study. All patients provided written informed consent before study entry.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi 2019;41:19-28. [PubMed]
- Spigel DR, Socinski MA. Rationale for chemotherapy, immunotherapy, and checkpoint blockade in SCLC: beyond traditional treatment approaches. J Thorac Oncol 2013;8:587-98. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006;24:4539-44. [Crossref] [PubMed]
- Früh M, De Ruysscher D, Popat S, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi99-105. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2021;22:51-65. [Crossref] [PubMed]
- Simon G, Turrisi A. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:324S-39S.
- Zugazagoitia J, Paz-Ares L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options. J Clin Oncol 2022;40:671-80. [Crossref] [PubMed]
- O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 2006;24:5441-7. [Crossref] [PubMed]
- von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999;17:658-67. [Crossref] [PubMed]
- Salven P, Ruotsalainen T, Mattson K, et al. High pre-treatment serum level of vascular endothelial growth factor (VEGF) is associated with poor outcome in small-cell lung cancer. Int J Cancer 1998;79:144-6. [Crossref] [PubMed]
- Fontanini G, Faviana P, Lucchi M, et al. A high vascular count and overexpression of vascular endothelial growth factor are associated with unfavourable prognosis in operated small cell lung carcinoma. Br J Cancer 2002;86:558-63. [Crossref] [PubMed]
- Li Q, Wu T, Jing L, et al. Angiogenesis inhibitors for the treatment of small cell lung cancer (SCLC): A meta-analysis of 7 randomized controlled trials. Medicine (Baltimore) 2017;96:e6412. [Crossref] [PubMed]
- Spigel DR, Townley PM, Waterhouse DM, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol 2011;29:2215-22. [Crossref] [PubMed]
- Pujol JL, Lavole A, Quoix E, et al. Randomized phase II-III study of bevacizumab in combination with chemotherapy in previously untreated extensive small-cell lung cancer: results from the IFCT-0802 trial†. Ann Oncol 2015;26:908-14. [Crossref] [PubMed]
- Ready NE, Pang HH, Gu L, et al. Chemotherapy With or Without Maintenance Sunitinib for Untreated Extensive-Stage Small-Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Phase II Study-CALGB 30504 (Alliance). J Clin Oncol 2015;33:1660-5. [Crossref] [PubMed]
- Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci 2011;102:1374-80. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. Journal of Clinical Oncology 2016;34:1448-54. [Crossref] [PubMed]
- Qin S, Li Q, Gu S, et al. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol 2021;6:559-68. [Crossref] [PubMed]
- Zhao H, Yao W, Min X, et al. Apatinib Plus Gefitinib as First-Line Treatment in Advanced EGFR-Mutant NSCLC: The Phase III ACTIVE Study (CTONG1706). J Thorac Oncol 2021;16:1533-46. [Crossref] [PubMed]
- Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [Crossref] [PubMed]
- Cheng H, Zong L, Kong Y, et al. Camrelizumab plus apatinib in patients with high-risk chemorefractory or relapsed gestational trophoblastic neoplasia (CAP 01): a single-arm, open-label, phase 2 trial. Lancet Oncol 2021;22:1609-17. [Crossref] [PubMed]
- Lan C, Shen J, Wang Y, et al. Camrelizumab Plus Apatinib in Patients With Advanced Cervical Cancer (CLAP): A Multicenter, Open-Label, Single-Arm, Phase II Trial. J Clin Oncol 2020;38:4095-106. [Crossref] [PubMed]
- Zhang B, Qi L, Wang X, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun (Lond) 2020;40:711-20. [Crossref] [PubMed]
- Liu C, Jia Q, Wei H, et al. Apatinib in patients with advanced chordoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2020;21:1244-52. [Crossref] [PubMed]
- Xie L, Xu J, Sun X, et al. Apatinib plus camrelizumab (anti-PD1 therapy, SHR-1210) for advanced osteosarcoma (APFAO) progressing after chemotherapy: a single-arm, open-label, phase 2 trial. J Immunother Cancer 2020;8:e000798. [Crossref] [PubMed]
- Zhong N, Zhuang W, Huang Q, et al. Apatinib inhibits the growth of small cell lung cancer via a mechanism mediated by VEGF, PI3K/Akt and Ki-67/CD31. J Cell Mol Med 2021;25:10039-48. [Crossref] [PubMed]
- Xu Y, Huang Z, Lu H, et al. Apatinib in patients with extensive-stage small-cell lung cancer after second-line or third-line chemotherapy: a phase II, single-arm, multicentre, prospective study. Br J Cancer 2019;121:640-6. [Crossref] [PubMed]
- Fan Y, Zhao J, Wang Q, et al. Camrelizumab Plus Apatinib in Extensive-Stage SCLC (PASSION): A Multicenter, Two-Stage, Phase 2 Trial. J Thorac Oncol 2021;16:299-309. [Crossref] [PubMed]
- Luo H, Zhang L, Yang B, et al. A randomized phase 2 trial of apatinib vs observation as maintenance treatment following first-line induction chemotherapy in extensive- stage small cell lung cancer. Invest New Drugs 2020;38:148-59. [Crossref] [PubMed]
- Liu Y, Hu X, Jiang J, et al. A Prospective Study of Apatinib in Patients with Extensive-Stage Small Cell Lung Cancer After Failure of Two or More Lines of Chemotherapy. Oncologist 2020;25:e833-42. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1—Update and clarification: From the RECIST committee. European Journal of Cancer 2016;62:132-7. [Crossref] [PubMed]
- Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 2007;25:2086-92. [Crossref] [PubMed]
- Schneider BJ. Management of recurrent small cell lung cancer. J Natl Compr Canc Netw 2008;6:323-31. [Crossref] [PubMed]
- Zhu YJ, Zhang HB, Liu YH, et al. Meta-analysis of the role of bevacizumab in extensive stage small cell lung cancer. Oncol Lett 2017;14:655-64. [Crossref] [PubMed]
- Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol 2020;21:645-54. [Crossref] [PubMed]
- Chung HC, Piha-Paul SA, Lopez-Martin J, et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients With Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J Thorac Oncol 2020;15:618-27. [Crossref] [PubMed]
- Ready NE, Ott PA, Hellmann MD, et al. Nivolumab Monotherapy and Nivolumab Plus Ipilimumab in Recurrent Small Cell Lung Cancer: Results From the CheckMate 032 Randomized Cohort. J Thorac Oncol 2020;15:426-35. [Crossref] [PubMed]
- Liang Q, Kong L, Du Y, et al. Antitumorigenic and antiangiogenic efficacy of apatinib in liver cancer evaluated by multimodality molecular imaging. Exp Mol Med 2019;51:1-11. [Crossref] [PubMed]
- Yang C, Qin S. Apatinib targets both tumor and endothelial cells in hepatocellular carcinoma. Cancer Med 2018;7:4570-83. [Crossref] [PubMed]
- Feng S, Wang H, Wang Y, et al. Apatinib induces 3-hydroxybutyric acid production in the liver of mice by peroxisome proliferator-activated receptor alpha activation to aid its antitumor effect. Cancer Sci 2019;110:3328-39. [Crossref] [PubMed]
- Cao Y, Ouyang T, Xiong F, et al. Efficacy of apatinib in patients with sorafenib-transarterial chemoembolization refractory hepatocellular carcinoma: a retrospective study. Hepatol Int 2021;15:1268-77. [Crossref] [PubMed]
- Simon GR, Wagner HAmerican College of Chest Physicians. Small cell lung cancer. Chest 2003;123:259S-71S. [Crossref] [PubMed]
- Sun D, Hou H, Zhang C, et al. The efficacy and safety of apatinib for refractory malignancies: a review and meta-analysis. Onco Targets Ther 2018;11:6539-54. [Crossref] [PubMed]
- Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol 2008;19:1955-61. [Crossref] [PubMed]
- Lee WJ, Lee JL, Chang SE, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol 2009;161:1045-51. [Crossref] [PubMed]
- Vincenzi B, Santini D, Russo A, et al. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist 2010;15:85-92. [Crossref] [PubMed]



