
A new N descriptor for non-small cell lung cancer: the classification based on anatomic location, number and ratio of metastatic lymph nodes
Introduction
Lung cancer is considered one of the most prevalent cancers worldwide, with non-small cell lung cancer (NSCLC) accounting for about 85% of all lung cancer cases (1,2). The average 5-year survival rate for NSCLC has only grown a little in the past few decades, indicating that reliable diagnostic and treatment strategies still have a long way to go (3). Appropriately assessing the tumor stage is vital to choose the optimal treatment strategy and anticipate the clinical outcomes of NSCLC patients. The International Association for the Study of Lung Cancer (IASLC) has proposed the tumor, node, metastasis (TNM) classification to evaluate the tumor stage and the 8th edition was released in 2015 (4). The T and M categories have been updated in comparison to the 7th edition, whereas the N classification has remained unchanged and is solely based on the anatomical location of metastatic lymph nodes (5). Several investigations have indicated that some limitations still exist in the N classification of the 8th TNM staging system. The clinical outcomes of patients stratified by the N classification tended to overlap, especially among those in N1 and N2 categories (6-9). Due to the inability of the N classification in predicting the prognosis of NSCLC patients, it is significant to update the current N classification and develop a more accurate N classification.
For most cancers, the anatomic location of metastatic lymph nodes (N-current) is not the only element determining the N classification, but the N-current is the only component that determines the N classification for lung cancer. The N classifications of other cancers, such as breast cancer, have introduced the number of positive lymph nodes to examined lymph nodes (N-ratio) and the number of positive lymph nodes (N-num) to the N classification (10). In the case of lung cancer, previous studies have investigated the reliability of the N-num and N-ratio, indicating that N-num and N-ratio can be more accurate prognostic factors than the current N classification (8,11-14). However, the combination of the N-current, N-num and N-ratio has not been investigated. It is still unclear whether the N-new classification, which combines N-current, N-num and N-ratio, could predict the prognosis of NSCLC patients better than the current N classification.
The goal of this study was to assess the prognostic accuracy and reliability of the existing N classification and the N-new classification, which may give new evidence and suggestions for the modification of the 9th TNM classification, based on a cohort with a significant number of N1 and N2 NSCLC patients. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-933/rc).
Methods
Patients enrollment
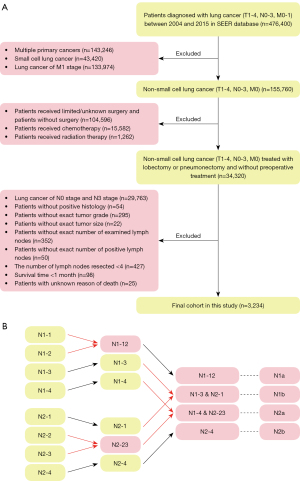
We screened for patients with N1 or N2 NSCLC from the 2004 to 2015 Surveillance, Epidemiology, and End Results (SEER) database. The data were consisted of deidentified patient-level information, with deidentified patients’ names and hospitals. Because SEER is a database available for the public, the ethical approval has been already finished by the committee of SEER database and additional ethical approval was not required. Our study is a retrospective study and individual consent for this retrospective analysis was waived. We excluded patients without comprehensive data of age, race, sex, primary site, tumor grade, laterality, histological type, surgical site, tumor size, the number of examined lymph nodes and the number of positive lymph nodes. Although the clinical guidelines recommend the patients undergo chemotherapy or radiotherapy in addition to the operation, we have excluded patients receiving chemotherapy or radiotherapy because of the data did not differentiate chemotherapy and radiotherapy implemented before or after the surgery. Due to chemotherapy and radiotherapy could downstage the lymph node stage, we believed that exclusion of all patients who received chemotherapy or radiotherapy was appropriate. Because the database only provided the number of resected lymph nodes as less than four and the other, we excluded patients with less than four resected lymph nodes, which met the criteria of TNM guidelines as much as possible (15,16). The detailed inclusion and exclusion criteria were presented in Figure 1. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data processing
We have enrolled eligible patients with exact information of age, race, sex, primary site, tumor grade, laterality, histological type, surgical site, tumor size, examined lymph nodes’ number and positive lymph node’s number, the use of chemotherapy and radiotherapy, survival time, survival status and cause of death in the SEER database. We aimed to stratify NSCLC patients into subgroups with different prognoses, so we used the X-tile software (version 3.6.1) to determine the optimal cut-off value (Figure S1). We first subdivided patients into N1 and N2 subgroups according to the N-stage classification. Next, we found that the optimal cut-off value was 0.21 for N-ratio and 4 for the N-num. N-ratio-low referred that the ratio of lymph nodes was less than 0.21, while N-ratio-high referred to the other. N-num-low referred to the N-num was less than four, while N-num-high was the other. Then in N1 and N2 we divided patients into four subgroups according to the combination of the above two categories: group-1 (N-num-low, N-ratio-low), group-2 (N-num-high, N-ratio-low), group-3 (N-num-low, N-ratio-high) and group-4 (N-num-high, N-ratio-high). Finally, the above four subgroups were subsequently divided in the N1 (N1-1, N1-2, N1-3 and N1-4) and N2 (N2-1, N2-2, N2-3 and N2-4) patients. We used cancer-specific survival as the clinical outcome in the present study. And the cancer-specific survival time was defined as the time from the date of diagnosis to the date of last follow-up or death from lung cancer.
The validation of N-new classification
We have stratified patients into different sT-stage according to the tumor size because the SEER database lacked the data of the 8th AJCC TNM categories, including sT1a (tumor size ≤1 cm), sT1b (1 cm < tumor size ≤2 cm), sT1c (2 cm < tumor size ≤3 cm), sT2a (3 cm < tumor size ≤4 cm), sT2b (4 cm < tumor size ≤5 cm), sT3 (5 cm < tumor size ≤7 cm) and sT4 (tumor size >7 cm). We have validated the N-new classification in various critical subgroups, such as patients of different sT-stage, patients with adenocarcinoma or squamous cell carcinoma, male or female patients and patients of different age groups. Moreover, we validated the N-new classification with the overall survival time of patients.
Statistical analysis
The t-test and Mann-Whitney U test were performed to compare the differences between continuous variables with normal distribution and continuous variables with non-normal distribution, respectively. The Chi-square test was used to compare the differences between categorical variables. The log-rank test and Kaplan-Meier method were applied to evaluate the differences among the cancer-specific survival of different subgroups. The univariable Cox regression analysis was performed to identify baseline characteristics with a prognostic impact. And the characteristic with a P value <0.05 was subsequently used in the multivariable Cox regression analysis. N-stage classification and N-new classification were introduced to different multivariable Cox regression analyses, respectively. Overall survival was defined as the time from treatment to death, while cancer-specific survival was defined as the time from treatment to death due to lung cancer. We validated the proportional hazard assumptions according to the results of Schoenfeld residual plots and log-log plots. Akaike information criterion (AIC), Harrell concordance index (C-index) and time-dependent receiver operating characteristic (ROC) curves were applied to analyze whether the multivariable Cox regression models performed well. The “compareC” R package and the “compare” function of the “timeROC” R package were used to evaluate the statistical difference between these two models. We analyzed the standardized net benefit of the N-new classification in predicting clinical outcomes via the decision curve analysis (DCA), which was performed using the R package “stdca”. P<0.05 was considered statistically significant and two-sided statistical tests were performed in this study based on R software (version 4.0.4, http://www.R-project.org).
Results
Clinical characteristics of patients
A total of 3,234 eligible NSCLC patients were identified from the SEER database, including 2,252 N1-stage patients (69.6%) and 982 N2-stage patients (30.4%), which were shown in Table 1. And the process of selecting patients for this study was presented in Figure 1A. Overall, 17.7%, 48.5% and 33.8% patients were with the age <65, 65–74 and >74 years, respectively; 55.1% patients were male and 44.9% patients were female. As for the histological type, adenocarcinoma (44.1%) was the most prevalent type and the squamous cell carcinoma (33.5%) was subsequent. Lobectomy/bilobectomy was performed in 2,526 patients (78.1%), extended lobectomy/bilobectomy was performed in 145 patients (4.5%), pneumonectomy was performed in 547 patients (16.9%) and extended pneumonectomy was performed in 16 patients (0.5%). The median number of examined lymph nodes was 11.00 [interquartile range (IQR), 7.00–17.00], and the median N-num was 2.00 (IQR, 1.00–3.00).
Table 1
| Characteristics | Overall, n=3,234 (100.0%) | N1, n=2,252 (69.6%) | N2, n=982 (30.4%) | P |
|---|---|---|---|---|
| Age, years, n (%) | ||||
| <65 | 574 (17.7) | 402 (17.9) | 172 (17.5) | 0.894 |
| 65–74 | 1,567 (48.5) | 1,085 (48.2) | 482 (49.1) | NA |
| >74 | 1,093 (33.8) | 765 (34.0) | 328 (33.4) | NA |
| Race, n (%) | ||||
| White | 2,733 (84.5) | 1,936 (86.0) | 797 (81.2) | 0.002 |
| Black | 271 (8.4) | 167 (7.4) | 104 (10.6) | NA |
| Other | 230 (7.1) | 149 (6.6) | 81 (8.2) | NA |
| Sex, n (%) | ||||
| Male | 1,781 (55.1) | 1,263 (56.1) | 518 (52.7) | 0.086 |
| Female | 1,453 (44.9) | 989 (43.9) | 464 (47.3) | NA |
| Primary site, n (%) | ||||
| Upper | 1,742 (53.9) | 1,208 (53.6) | 534 (54.4) | 0.677 |
| Middle | 141 (4.4) | 98 (4.4) | 43 (4.4) | NA |
| Lower | 1,124 (34.8) | 778 (34.5) | 346 (35.2) | NA |
| Overlap | 118 (3.6) | 84 (3.7) | 34 (3.5) | NA |
| Main bronchus | 69 (2.1) | 53 (2.4) | 16 (1.6) | NA |
| NOS | 40 (1.2) | 31 (1.4) | 9 (0.9) | NA |
| Tumor grade, n (%) | ||||
| I | 194 (6.0) | 134 (6.0) | 60 (6.1) | 0.965 |
| II | 1,341 (41.5) | 929 (41.3) | 412 (42.0) | NA |
| III | 1,606 (49.7) | 1,125 (50.0) | 481 (49.0) | NA |
| IV | 93 (2.9) | 64 (2.8) | 29 (3.0) | NA |
| Laterality, n (%) | ||||
| Left | 1,506 (46.6) | 1,045 (46.4) | 461 (46.9) | 0.814 |
| Right | 1,727 (53.4) | 1,206 (53.6) | 521 (53.1) | NA |
| Histology, n (%) | ||||
| Adenocarcinoma | 1,425 (44.1) | 938 (41.7) | 487 (49.6) | <0.001 |
| Squamous cell carcinoma | 1,085 (33.5) | 832 (36.9) | 253 (25.8) | NA |
| Others | 724 (22.4) | 482 (21.4) | 242 (24.6) | NA |
| Surgical site, n (%) | ||||
| Lobe or bilobectomy | 2,526 (78.1) | 1,722 (76.5) | 804 (81.9) | <0.001 |
| Lobe or bilobectomy extended | 145 (4.5) | 123 (5.5) | 22 (2.2) | NA |
| Pneumonectomy | 547 (16.9) | 399 (17.7) | 148 (15.1) | NA |
| Pneumonectomy extended | 16 (0.5) | 8 (0.4) | 8 (0.8) | NA |
| sT-stage, n (%) | ||||
| T1a | 43 (1.3) | 33 (1.5) | 10 (1.0) | 0.227 |
| T1b | 482 (14.9) | 320 (14.2) | 162 (16.5) | NA |
| T1c | 793 (24.5) | 542 (24.1) | 251 (25.6) | NA |
| T2a | 653 (20.2) | 462 (20.5) | 191 (19.5) | NA |
| T2b | 480 (14.8) | 352 (15.6) | 128 (13.0) | NA |
| T3 | 479 (14.8) | 337 (15.0) | 142 (14.5) | NA |
| T4 | 304 (9.4) | 206 (9.1) | 98 (10.0) | NA |
| Examined lymph nodes, median [IQR] | 11.00 [7.00, 17.00] | 11.00 [7.00, 17.00] | 11.00 [7.00, 16.00] | 0.121 |
| Positive lymph nodes, median [IQR] | 2.00 [1.00, 3.00] | 1.00 [1.00, 3.00] | 3.00 [1.00, 5.00] | <0.001 |
Only one patient with N1 stage was recorded as “Not_paired_site” for “Laterality” in the database, which was not presented in the table. NA, not applicable; NOS, not otherwise specified; IQR, interquartile range.
The prognostic value of the N1-new and N2-new classifications
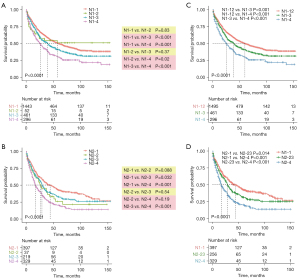
In order to develop a more reliable and effective N category in N1&N2 patients, we enrolled three different variables: the current N staging category (N-stage), N-ratio and the N-num. According to the optimal cut-off value determined by the X-tile software, we have stratified N1 and N2 patients into four subgroups, respectively. N1-1 (N1 stage, N-num <4 and N-ratio <0.21), N1-2 (N1 stage, N-num ≥4 and N-ratio <0.21), N1-3 (N1 stage, N-num <4 and N-ratio ≥0.21) and N1-4 (N1 stage, N-num ≥4 and N-ratio ≥0.21) were four subgroups in N1 patients, which were belonged to the original N1-new classification. N2-1 (N2 stage, N-num <4 and N-ratio <0.21), N2-2 (N2 stage, N-num ≥4 and N-ratio <0.21), N2-3 (N2 stage, N-num <4 and N-ratio ≥0.21) and N2-4 (N2 stage, N-num ≥4 and N-ratio ≥0.21) were other four subgroups in N2 patients, which were belonged to the original N2-new classification. Next, we evaluated the prognostic value of the above two new classifications. The Kaplan-Meier curves indicated that the original N1-new and N2-new classifications can almost stratify N1 and N2 patients into four distinct subgroups with different prognoses, respectively (Figure 2A,2B). However, the clinical outcomes of patients in N1-2 subgroup were similar to patients in N1-1 and N1-3 (N1-2 vs. N1-1, P=0.83; N1-2 vs. N1-3, P=0.37). And the prognosis of patients in N2-2 subgroup was also not distinctive compared with other three subgroups (N2-2 vs. N2-1, P=0.088; N2-2 vs. N2-3, P=0.54; N2-2 vs. N2-4, P=0.19). To make the original N1-new and N2-new classifications more reliable and accurate, we combined the N1-1 and N1-2 subgroups in N1 patients, and we combined N2-2 and N2-3 subgroups in N2 patients. Finally, the updated N1-new category was composed of N1-12, N1-3 and N1-4, while the updated N2-new category was composed of N2-1, N2-23 and N2-4 (Figure 2C,2D). And the differences in the cancer-specific survival between every two subgroups in N1 and N2 patients were statistically significant.
The survival significance of the N-new classification
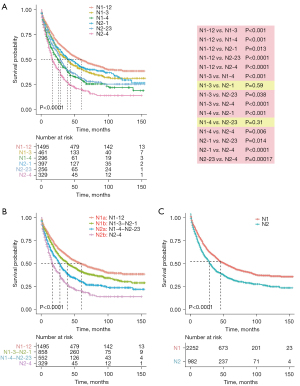
We combined the N1-new and N2-new classifications into the original N-new classification, aiming to develop a novel N staging system that can precisely stratify patients into subgroups with diverse clinical outcomes. We compared the survival probability of each two subgroups in our cohort, which revealed that the survival difference was statistically significant in all comparisons except in two pairs (Figure 3A). The Kaplan-Meier curves of N1-3&N2-1 (P=0.59) and N1-4&N2-23 (P=0.31) were close to each other. Herein, we grouped the categories whose curves overlapped each other, including N1-3&N2-1 and N1-4&N2-23, into two individual subgroups and finally got the N-new classification. The log-rank test indicated that four subgroups of the N-new classification had a well-distributed prognosis (Figure 3B). We renamed the four subgroups as N1a (N1-12), N1b (N1-3-N2-1), N2a (N1-4-N2-23) and N2b (N2-4) (Figure 1B). Overall, 46.3% patients were in the N1a subgroup, 26.5% of patients were in N1b subgroup, 17.1% of patients were in N2a subgroup and 10.1% of patients were in N2b subgroup. Our results suggested that the unfavorable clinical outcomes were closely associated with increased N-new stages, the combination of N-stage, N-num and N-ratio. The 5-year CSS rates were 49.7%, 41.4%, 30.4% and 20.4% for N1a, N1b, N2a and N2b, respectively. And the differences of the survival probability between each pair of subgroups were also significant (N1a vs. N1b, P=0.00022; N1a vs. N2a, P<0.0001; N1a vs. N2b, P<0.0001; N1b vs. N2a, P<0.0001; N1b vs. N2b, P<0.0001; N2a vs. N2b, P=0.00013). Besides, the 5-year CSS rates were 45.2% and 32.2% for N1 and N2, respectively (Figure 3C).
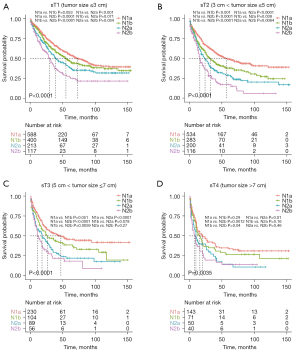
Furthermore, we analyzed the prognostic impact of the N-new classification, aiming to validate its reliability in different subgroups. First, we stratify patients based on the histological type: adenocarcinoma and squamous cell carcinoma. The Kaplan-Meier curves revealed that each pair of N-new classifications had a significant difference in clinical outcomes in patients with adenocarcinoma except between N1a and N1b (Figure S2A). And the results indicated significant differences between each pair of N-new classifications in patients with squamous cell carcinoma except between N2a and N2b (Figure S2B). Then we analyzed the efficacy of N-new classification in male and female patients, respectively. Our results indicated that each pair of N-new categories was statistically different in the male and female patients, respectively (Figure S2C,S2D). Next, we validate our N-new classification in patients with different tumor size, including sT1 stage (tumor size ≤3 cm), sT2 stage (3 cm < tumor size ≤5 cm), sT3 stage (5 cm < tumor size ≤7 cm) and sT4 stage (tumor size >7 cm). For sT1 stage, the curves of N1a and N1b overlapped, while the survival curves of other pairs had clear differences (Figure 4A). For sT2 stage, the survival curves revealed significant differences between each pair of N-new classifications (Figure 4B). For sT3 stage, the results demonstrated that N2a and N2b did not statistically differ (Figure 4C). And for sT4 stage, the survival curves of the three pairs were not statistically different, including N1a/N1b, N1b/N2a and N2a/N2b (Figure 4D). Furthermore, we also found that the N-new classification could perform well in patients with different age subgroups in predicting the prognosis (Figure S3A-S3C). And it can also be used to predict the overall survival time of NSCLC patients (Figure S3D). To sum up, the tendency toward deterioration of cancer-specific survival from N1a to N2b subgroups could be significantly seen.
The predictive efficacy of N-new classification
We performed the multivariable Cox regression analyses and the results indicated that N-new and N-stage classifications were both the independent prognostic factor (Table 2). The DCA was conducted to analyze the efficacy of the N-new classification in predicting prognosis, which showed that N-new classification provided a larger net benefit for prognosis prediction (Figure 5A). And we found that the N-new classification performed better than the AJCC 8th N-stage classification to stratify patients with distinct clinical outcomes, with smaller AIC and higher C-index (P<0.001). The time-dependent ROC curves showed that the multivariable Cox regression model with the N-new classification had a higher area under the curves (AUCs) than the other (Figure 5B,5C). The 3- and 5-year P values of time-dependent ROC curves were less than 0.001 between these two models.
Table 2
| Characteristics | Univariable | Multivariable-new | Multivariable-current | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |||
| N-new | ||||||||
| N1a | Reference | Reference | NA | |||||
| N1b | 1.248 (1.109–1.404) | <0.001 | 1.328 (1.179–1.495) | <0.001 | NA | NA | ||
| N2a | 1.663 (1.463–1.891) | <0.001 | 1.746 (1.534–1.987) | <0.001 | NA | NA | ||
| N2b | 2.31 (1.991–2.681) | <0.001 | 2.28 (1.962–2.65) | <0.001 | NA | NA | ||
| N-stage | ||||||||
| N1 | Reference | NA | Reference | |||||
| N2 | 1.406 (1.275–1.552) | <0.001 | NA | NA | 1.492 (1.351–1.647) | <0.001 | ||
| Age, years | ||||||||
| <65 | Reference | Reference | Reference | |||||
| 65–74 | 1.315 (1.146–1.509) | <0.001 | 1.349 (1.173–1.551) | <0.001 | 1.32 (1.148–1.517) | <0.001 | ||
| >74 | 1.593 (1.381–1.838) | <0.001 | 1.73 (1.491–2.007) | <0.001 | 1.71 (1.474–1.983) | <0.001 | ||
| Race | ||||||||
| White | Reference | NA | NA | |||||
| Black | 0.981 (0.824–1.166) | 0.824 | NA | NA | NA | NA | ||
| Others | 1.077 (0.903–1.285) | 0.407 | NA | NA | NA | NA | ||
| Sex | ||||||||
| Male | Reference | Reference | Reference | |||||
| Female | 0.75 (0.682–0.825) | <0.001 | 0.753 (0.683–0.83) | <0.001 | 0.769 (0.697–0.847) | <0.001 | ||
| Marital status | ||||||||
| Married | Reference | NA | NA | |||||
| Divorced or separated | 1.084 (0.942–1.247) | 0.263 | NA | NA | NA | NA | ||
| Unmarried | 1.097 (0.945–1.274) | 0.222 | NA | NA | NA | NA | ||
| Widowed | 1.135 (0.999–1.29) | 0.052 | NA | NA | NA | NA | ||
| Primary site | ||||||||
| Upper | Reference | Reference | Reference | |||||
| Middle | 0.939 (0.735–1.199) | 0.611 | 0.992 (0.776–1.269) | 0.95 | 0.973 (0.761–1.243) | 0.824 | ||
| Lower | 1.118 (1.009–1.238) | 0.033 | 1.063 (0.958–1.178) | 0.249 | 1.087 (0.981–1.206) | 0.113 | ||
| Overlap | 1.575 (1.245–1.993) | <0.001 | 1.361 (1.069–1.733) | 0.012 | 1.396 (1.097–1.777) | 0.007 | ||
| Main bronchus | 1.663 (1.216–2.274) | 0.001 | 1.525 (1.099–2.116) | 0.012 | 1.464 (1.055–2.031) | 0.023 | ||
| NOS | 1.157 (0.751–1.784) | 0.508 | 1.075 (0.695–1.664) | 0.744 | 1.17 (0.756–1.81) | 0.481 | ||
| Tumor grade | ||||||||
| I | Reference | Reference | Reference | |||||
| II | 1.653 (1.292–2.115) | <0.001 | 1.578 (1.231–2.021) | <0.001 | 1.621 (1.266–2.078) | <0.001 | ||
| III | 2.259 (1.772–2.88) | <0.001 | 2.01 (1.574–2.568) | <0.001 | 2.115 (1.656–2.701) | <0.001 | ||
| IV | 2.204 (1.545–3.143) | <0.001 | 1.936 (1.354–2.769) | <0.001 | 1.883 (1.317–2.692) | 0.001 | ||
| Laterality | ||||||||
| Left | Reference | NA | NA | |||||
| Right | 1.012 (0.921–1.112) | 0.81 | NA | NA | NA | NA | ||
| Histology | ||||||||
| Adenocarcinoma | Reference | NA | NA | |||||
| Squamous cell carcinoma | 1.081 (0.97–1.204) | 0.158 | NA | NA | NA | NA | ||
| Others | 0.986 (0.872–1.114) | 0.816 | NA | NA | NA | NA | ||
| Surgical site | ||||||||
| Lobe or bilobectomy | Reference | Reference | Reference | |||||
| Lobe or bilobectomy extended | 1.335 (1.076–1.657) | 0.009 | 1.356 (1.091–1.684) | 0.006 | 1.382 (1.112–1.719) | 0.004 | ||
| Pneumonectomy | 1.347 (1.192–1.522) | <0.001 | 1.145 (0.997–1.316) | 0.056 | 1.185 (1.031–1.361) | 0.017 | ||
| Pneumonectomy extended | 2.156 (1.249–3.724) | 0.006 | 1.511 (0.863–2.646) | 0.149 | 1.658 (0.95–2.894) | 0.075 | ||
| sT-stage | ||||||||
| T1 | Reference | Reference | Reference | |||||
| T2 | 1.36 (1.218–1.518) | <0.001 | 1.303 (1.164–1.457) | <0.001 | 1.298 (1.16–1.453) | <0.001 | ||
| T3 | 1.528 (1.325–1.762) | <0.001 | 1.35 (1.164–1.564) | <0.001 | 1.341 (1.157–1.553) | <0.001 | ||
| T4 | 2.102 (1.794–2.463) | <0.001 | 1.741 (1.471–2.062) | <0.001 | 1.768 (1.494–2.091) | <0.001 | ||
| C-index | NA | NA | 0.652 (0.638–0.666) | 0.639 (0.625–0.653) | ||||
| AIC | NA | NA | 25,659.55 | 25,734.11 | ||||
NA, not applicable; NOS, not otherwise specified; AIC, Akaike information criterion.

Discussion
It is critical to determine the optimal treatment strategy and predict the clinical outcomes for NSCLC patients using the TNM staging system. However, the existing N classification has remained unchanged for many years, and it is unsatisfactory to stratify NSCLC patients, especially N1 and N2 patients. For example, NSCLC patients with skip metastases (pN0N2), also known as N2 without N1 involvement, have been considered as a distinct subgroup of N2 patients with a better prognosis than pN2 patients (17). The current N classification, on the other hand, can only classify pN0N2 NSCLC patients into the N2 subgroup, which usually has a poorer prognosis than the N1 subgroup. Due to the limitation of the current N classification, a more reliable and accurate N-classification is urgently needed for individualized precision therapy.
In addition to the 8th edition of the TNM classification, a recent study found that the N-num had a substantial impact on the clinical outcomes of NSCLC patients, suggesting that N-num might be considered a possible prognostic indicator (7). Fukui et al. (18) have revealed that elevated N-num was closely related to poor prognosis and may represent the better prognoses of skip or single-station nodal metastases. Furthermore, Chen et al. (12) also demonstrated that the N-ratio could be used as an independent prognostic factor, which is especially effective in the N1 NSCLC patients (19). However, according to the results of the ROC curves, the differences in AUCs between N-ratio and current N staging were not statistically significant, indicating that N-ratio was not adequate for the only classification of NSCLC patients (19). And the current N classification still has its value and has been used as a criterion for a long time. Thus, the current N classification is included in the N-new classification. In this work, we created the N-new classification by combining the existing N classification, N-num, and N-ratio, and assessed its accuracy and reliability using a large population-based cohort. We specifically focused on N1 and N2 NSCLC patients because of the existing N classification’s poor performance in classifying prognostically heterogenous N1 and N2 patients. We initially determined the optimal cut-off value of N-num and N-ratio based on the cancer-specific survival of NSCLC patients. Next, based on the combinations of the N-num and N-ratio, we stratified N1 and N2 patients into four different subgroups, respectively (N1-1/2/3/4, N2-1/2/3/4). The results revealed that the difference in the survival curves of N1-1 and N1-2 was the least significant in N1 patients, whereas the difference in the survival curves of N2-2 and N2-3 was the least significant in N2 patients. We subsequently integrated the aforementioned two pairs of subgroups and discovered the survival curves in N1 and N2 subgroups were widely distributed, suggesting the N1 and N2 subgroups were prognostically heterogenous and the combination of N-num & N-ratio functioned well.
Considering the heterogeneity of N1 and N2 patients, we believed that the N-new classification needs to be used to differentiate N1 and N2 patients rather than classifying N1 or N2 patients independently. Herein, we combined the three categories of N1 patients and the other three categories of N2 patients and found that the survival curves of two pairs of subgroups overlapped (N1-3 and N2-1, N1-4 and N2-23), indicating the heterogeneity in these N-new groupings. Thus, we combined these two pairs of subgroups and finally subdivided N1 and N2 patients into four distinct subgroups (N1a, N1b, N2a and N2b). Several studies have incorporated N-num or N-ratio into the current N classification, while other investigations have used both N-num and N-ratio as the novel N classification (7,11,13). Li et al. (11) independently used the N-num and N-ratio classifications, showing that both N-num and N-ratio classifications had a higher C-index than the current N classification. And N-num classification had a higher C-index than the N-ratio. However, no study has investigated the combination of all these three prognostic variables and evaluated its accuracy and reliability in NSCLC patients. Our study has identified four subgroups based on the combination of current N classification, N-num and N-ratio, which may take full advantage of all potential lymph node characteristics, to determine whether the combination of all these parameters can be used as a more reliable N classification. And the Kaplan-Meier curves revealed that the survival curves of the N-new classification were proportional and the differences between all the pairs were significant. Compared with a prior study that stratifies N1 and N2 patients into three subgroups, we created a more precise and widely utilized staging system for nodal status (11). The aforesaid findings suggested that we have developed a reliable N-new classification and its performance was satisfactory to stratify N1 and N2 patients into subgroups with different clinical outcomes.
In order to further validate the performance of the N-new classification in N1 and N2 NSCLC patients, we analyzed the survival curves determined by the N-new classification across each cancer type, gender, tumor size and age category. The N-new classification performed well in each gender category, indicating gender had no bearing on the performance of the N-new classification. In terms of the cancer type, our results showed that survival curves of N1a and N1b overlapped in lung adenocarcinoma, whereas survival curves of N2a and N2b overlapped in lung squamous cell carcinoma. These findings showed that the N-new classification should be revised to account for the impact of cancer type. Next, we evaluated the accuracy of the N-new classification in different subgroups based on the tumor size. We discovered that the N-new classification performed poorly when tumor size increased, which might be due to the small number of cases. The poorer performance may possibly be linked to the fact that patients with larger tumor had worse clinical outcomes, regardless of the status of lymph nodes. Moreover, we found that survival curves of N1b and N2a overlapped each other in patients younger than 65 years, whereas survival curves of N2a and N2b overlapped each other in the other. As a result, age may be an important aspect to consider when the N-new classification is revised in the future. We also validated the N-new classification according to the overall survival of NSCLC patients, and the results showed that our N-new classification can effectively stratify patients into subgroups with varying prognoses. According to the multivariable Cox regression analysis, the current N classification and the N-new classification were both independent prognostic factors. Besides, when compared to the current N classification, the N-new classification had smaller AIC, higher C-index, larger net benefit and higher AUCs, indicating a better performance of the N-new classification.
According to prior studies, the number of resected lymph nodes has a substantial impact on surgical quality and tumor accurate staging (20,21). Therefore, determining an optimal number of the resected lymph nodes is vital before assessing the NSCLC patients’ prognosis using the N-new classification. However, the number of the resected lymph nodes was not defined by the current guidelines and the exact number was controversial. Saji et al. have shown that 10 removal lymph nodes might have a substantial impact on patients’ clinical outcomes, whereas Liang et al. demonstrated that 16 examined lymph nodes could be an optimal threshold for nodal staging and related to the prognosis of NSCLC patients (20,22). In addition, Xu et al. (23) have set 6 as the optimal number of the resected lymph nodes in their recent study. Due to the available data in the SEER database and the lack of guidelines suggesting the optimal number of resected lymph nodes, we selected patients with the removal of more than 4 lymph nodes for further analysis.
Despite the fact that the N-new classification performed well in stratifying heterogenous N1 and N2 patients, some limitations still existed in our study. First, although the patients were from a real-world and population-based cohort, our study was retrospective and may be restricted by the single database. The multi-center prospective studies may be helpful to validate our results in the future. Second, certain SEER database data, like as the number of resected lymph nodes, was incomplete. The database made no distinction between nodal fragments and entire lymph nodes. So patients may not be classified precisely and the number of the removal lymph nodes may be estimated, resulting in bias in the study. Besides, the SEER database lacked adequate information of the resected lymph nodes, and Maeshima et al. (24) found that insufficient examination of lymph nodes in some important stations may lead to a potential weakness. Moreover, we excluded individuals who received chemotherapy and radiotherapy because the SEER database could not differentiate therapy implemented before or after the surgery. As a result, we were unable to assess the new classification’s effectiveness in patients undergoing chemotherapy or radiation. Third, we validated the N-new classification in subgroups determined by a variety of clinical characteristics, revealing that some characteristics may be considered for future revision of the N-new classification. Fourth, we used detailed characteristics of the lymph nodes and some information can be only obtained after the surgery. Only patients who had a procedure with systematic mediastinal lymphadenectomy and no neoadjuvant therapy were eligible for the N-new classification.
Conclusions
In summary, compared with the 8th edition of the N classification, our study revealed that the N-new classification (combination of the current N classification, N-num and N-ratio) could stratify N1 and N2 NSCLC patients into subgroups with a prognostic difference, which can be worthful in the forthcoming 9th edition of the TNM classification. Our N-new classification, in particular, can only be applied postoperatively and only to N1 and N2 cases. The multi-center prospective studies are warranted to validate the reliability of the N-new classification and determine more precise cut-off values of the N-num and N-ratio in the future.
Acknowledgments
We thank the SEER tumor registries for providing open access to the database.
Funding: The study was funded by Institutional Fundamental Research Funds (No. 2018PT32033) and the Ministry of Education Innovation Team Development Project (No. IRT-17R10).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-933/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-21-933/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Zhou B, Zang R, Zhang M, et al. Worldwide burden and epidemiological trends of tracheal, bronchus, and lung cancer: A population-based study. EbioMedicine 2022;78:103951. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:300-11.
- Travis WD, Giroux DJ, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the inclusion of broncho-pulmonary carcinoid tumors in the forthcoming (seventh) edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2008;3:1213-23. [Crossref] [PubMed]
- Sakao Y, Okumura S, Mun M, et al. Prognostic heterogeneity in multilevel N2 non-small cell lung cancer patients: importance of lymphadenopathy and occult intrapulmonary metastases. Ann Thorac Surg 2010;89:1060-3. [Crossref] [PubMed]
- Katsumata S, Aokage K, Ishii G, et al. Prognostic Impact of the Number of Metastatic Lymph Nodes on the Eighth Edition of the TNM Classification of NSCLC. J Thorac Oncol 2019;14:1408-18.
- Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol 2011;6:310-8. [Crossref] [PubMed]
- Jonnalagadda S, Arcinega J, Smith C, et al. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer 2011;117:4724-31. [Crossref] [PubMed]
- Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:290-303.
- Li F, Yuan L, Zhao Y, et al. Comparison of Two Proposed Changes to the Current Nodal Classification for Non—small Cell Lung Cancer Based on the Number and Ratio of Metastatic Lymph Nodes. Chest 2021;160:1520-33. [Crossref] [PubMed]
- Chen W, Zhang C, Wang G, et al. Feasibility of nodal classification for non-small cell lung cancer by merging current N categories with the number of involved lymph node stations. Thorac Cancer 2019;10:1533-43. [Crossref] [PubMed]
- Matsuguma H, Oki I, Nakahara R, et al. Proposal of new nodal classifications for non-small-cell lung cancer based on the number and ratio of metastatic lymph nodes. Eur J Cardiothorac Surg 2012;41:19-24. [PubMed]
- Rusch VW, Giroux DJ. Nodal staging in lung cancer: lymph node location or number? J Thorac Oncol 2011;6:237-8. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Li X, Li X, Fu X, et al. Survival benefit of skip metastases in surgically resected N2 non-small cell lung cancer: A multicenter observational study of a large cohort of the Chinese patients. Eur J Surg Oncol 2020;46:1874-81. [Crossref] [PubMed]
- Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol 2006;1:120-5. [Crossref] [PubMed]
- Qiu C, Dong W, Su B, et al. The prognostic value of ratio-based lymph node staging in resected non-small-cell lung cancer. J Thorac Oncol 2013;8:429-35. [Crossref] [PubMed]
- Liang W, He J, Shen Y, et al. Impact of Examined Lymph Node Count on Precise Staging and Long-Term Survival of Resected Non-Small-Cell Lung Cancer: A Population Study of the US SEER Database and a Chinese Multi-Institutional Registry. J Clin Oncol 2017;35:1162-70. [Crossref] [PubMed]
- Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol 2012;30:2823-8. [Crossref] [PubMed]
- Saji H, Tsuboi M, Yoshida K, et al. Prognostic impact of number of resected and involved lymph nodes at complete resection on survival in non-small cell lung cancer. J Thorac Oncol 2011;6:1865-71. [Crossref] [PubMed]
- Xu L, Su H, She Y, et al. Which N Descriptor Is More Predictive of Prognosis in Resected Non-small Cell Lung Cancer: The Number of Involved Nodal Stations or the Location-Based Pathological N Stage? Chest 2021;159:2458-69. [Crossref] [PubMed]
- Maeshima AM, Tsuta K, Asamura H, et al. Prognostic implication of metastasis limited to segmental (level 13) and/or subsegmental (level 14) lymph nodes in patients with surgically resected nonsmall cell lung carcinoma and pathologic N1 lymph node status. Cancer 2012;118:4512-8. [Crossref] [PubMed]


