
Kisspeptins and norepinephrine regulate different G-protein-coupled receptor signaling pathways
In addition to circulating tumor DNA (ctDNA)-directed liquid biopsy assays in cancer patients, combination of ctDNA with other liquid biopsy analysis detectable in the blood of lung cancer patients, such as circulating tumor cells, extracellular vesicles, miRNAs, or proteins, may permit the development of composite biomarker tests (1). The KISS1 gene encodes KISS1, a protein processed in serum into active smaller peptides, named kisspeptins (KPs), which have been reported to exhibit anti-metastatic function in melanoma, as well in other primary malignancies. However, a tumor promoter function has also been reported, based on several elements, such as the absence or presence of other signaling molecules that might facilitate suppressor or promoter cancer pathways (2). Corno and Perego (3) have reviewed the role of KISS1 as a metastasis suppressor in regulation of metastasis and response to antitumor agents. Loss of reduced KISS1 expression is partly due to the relation of promoter hypermethylation in the development of metastasis in several types of cancer. Nevertheless, controversial findings have surfaced, showing that KISS signaling leads to cisplatin resistance in triple breast cancer, and cisplatin apoptosis in head and neck squamous cell carcinoma [see Fig. 3 in Como, Perego. Drug Resistance Updates 2019 (3)]. In this issue of Translational Lung Cancer Research, Perego and colleagues (4) communicate the presence of KISS1-derived peptide levels in liquid biopsies from 60 non-small cell lung cancer (NSCLC) patients examined by ELISA assay. The KISS1 levels in serum were increased in NSCLC patients compared to healthy donors. In addition, KISS1 mRNA levels increased in cancer cell lines treated with azacitidine and cisplatin, suggesting that KISS1-derived peptides could predict response to cancer therapy and become a biomarker in NSCLC (4). However, disparities in KISS1 protein levels have been found in other studies where they are higher in normal tissue rather than NSCLC tissue, but in general, elevated KISS1 expression is associated with better prognosis (5).
KISS1 was first described as a metastasis suppressor gene in melanoma. KISS1 encodes a precursor peptide of 145 amino-acids, which is processed by proteolytic cleavage into shorter peptides, KPs, KP-10, KP-13, KP-14, and KP-54 (metastin). KPs bind to G-protein-coupled receptor 54 (GPR54), also called KISS-1R, that activates the G-Proteins Gαq/11 (6) (Figure 1). GPR54 is highly distributed in the pancreas, placenta, pituitary gland, and spinal cord. It is relatively abundant in the hypothalamus, limbic system, and basal ganglia, as well as in the spleen, peripheral blood leukocytes, testis, and lymph node (7). In the hypothalamus, KISS1/GPR54 is coupled to Gαq/11, which activates phospholipase C (PLC) and leads to the hydrolysis of phosphatidylinositol-4, 5-bisphosphate. This, in turn, results in the production of inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) that act as two different potential “second messengers”. IP3 can contribute to the increase in intracellular Ca2+. DAG3 leads to the activation of protein kinase C (PKC), extracellular signal-regulated kinases 1/2 (ERK1/2) and p38 phosphorylation (Figure 1). β-arrestin 1 and 2, can promote activation or inhibition of ERK1/2, respectively (5) (Figure 1). KISS1 plays an essential role in inhibiting insulin secretion in mice liver. When the blood glucose level is downregulated in the body, the secretion of glucagon is increased, acting upon the glucagon receptor in the liver. The cyclic adenosine 3',5'-monophosphate (cAMP)-PKA-cAMP response element-binding protein (CREB) is triggered and the transcription of KISS1 gene is activated, resulting in suppression of insulin secretion (7) (Figure 1). Interestingly, activation of Gs-coupled receptors by epinephrine or glucagon stimulation increases large tumor suppressor Kinase 1/2 (LATS1/2), inhibiting Yes Associated Protein (YAP) function in the Hippo pathway (8). In contrast, activation of Gαq/11-coupled receptors by lysophosphatidic acid (LPA) or sphingosine 1-phosphatase (SIP) inhibits LATS1/2 kinases, resulting in YAP activation (8). Therefore, it is tempting to posit that KPs, through activating G proteins Gαq/11, could activate YAP and YAP target genes, such as CYR61, TGF, and angiomotin like 2 (AMOTL2) (Figure 1). Of interest is the fact that different roles of the Hippo-YAP pathways are mediated by different G-protein-coupled receptors and their corresponding extracellular ligands (8).
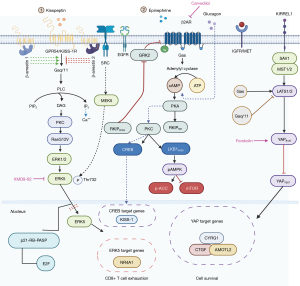
Psychological stress is associated with poor prognosis in cancer patients and promotes lung carcinogenesis progression in mice (9). In addition, stress hormones-activated β2-adrenergic receptors cause resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant NSCLC (10). It has recently been described that chronic stress increases KP levels and activates GPR54 to stimulate lung cancer progression by enhancing the exhaustion of CD8+ T cells in a mouse model (11). Evidence points out that cancer cells take advantage of the neurotransmitters-initiated signaling pathway to activate uncontrolled proliferation and dissemination, therefore, the KP/GPR54 axis. Neurotransmitters released from peripheral and autonomic nerves based on their specific chemical structure are classified into three categories: (I) amino acids, such as acetylcholine, glutamate, glycine, and gamma-aminobutyric acid (GABA); (II) biogenic amines, including dopamine, norepinephrine (NE), epinephrine, and serotonin; (III) peptidergic neurotransmitters (or neuropeptides), such as substance P, and neuropeptide Y (NPY), are well-established (12), and we can now also consider KPs. KP/GPR54 signaling was significantly upregulated by psychological stress, negatively regulating cancer immunosurveillance by promoting CD8+ T cell exhaustion (11). Both KISS1 and GPR54 accumulated in the hypothalamus of acute restraint mice, in addition to increased GPR54 expression in splenic T cells. KP serum levels were also elevated in acute restraint mice (11). Furthermore, the authors observed increased tumor growth and enhanced GPR54 expression in tumor infiltrating lymphocytes and higher serum levels of KP 1 in acute restraint-treated xenograft mice (11). They reviewed the Cancer Genome Atlas (TCGA) and noted that increased expression of GRP54 in human lung cancer tissues and higher GPR54 expression in lung adenocarcinoma patients correlates with poor prognosis. Moreover, GPR54 expression was negatively associated with CD8+ T cell infiltration in lung adenocarcinoma, kidney, renal clear cell carcinoma, lung squamous cell carcinoma, and testicular germ cell tumors. Only the expression of GPR54 was linked to CD8+ T cell infiltration in lung cancer, despite the detection of Grin1 (glutamate receptor), Ptger1 (prostaglandin E receptor 1), Hrh2 (histamine receptor), and Tacr1 (substance P receptor) (11). In a model of subcutaneously implanted Lewis lung cancer (LLC) cells in mice, GPR54 deficiency (Gpr54−/− mice) reduced subcutaneous tumor growth. Furthermore, in comparison with the control group, KP-10 inhibited the proliferation of CD8+ T cells. Intriguingly, the nuclear receptor subfamily 4 group A (NR4A), a key mediator of T cell dysfunction, was activated via GPR54 through activation of ERK5. Mice bearing LLC tumors were treated with the ERK5 inhibitor (XMD8-92), leading to a reduction in tumor burden, but the effect of XMD8-92 disappeared when GPR54 was ablated (11). Overall, the results support that GPR54 or ERK5 depletion increases T cell function. Intracellular calcium is essential for Gq-coupled GPCRs, as well as ERK5 phosphorylation (Figure 1). The study of Zhang et al. (11) shows that ERK5-mediated NR6A1 activation is involved in KP/GPR54 T cell dysfunction. Oncogenic BRAF (BRAF V600E) and MEK5 enhance ERK5 phosphorylation at Thr 732 for melanoma growth in vitro and in vivo (13). Also, mitogens and stress factors (activating SRC for example) can lead to the activation of upstream activators of MEK5 (14,15) (Figure 1). Intriguingly, ERK5 knockdown or inhibition with XMD8-92 in melanoma xenografts promotes cellular senescence with p21 expression (16). Cell stress (such as that caused by KRAS G12V) induces p21 activation that can arrange cell cycle arrest and immunosurveillance through retinoblastoma (RB) hypo phosphorylation. Furthermore, p21 induction triggers the p21-associated secretory phenotype (PASP) including secretion of C-X-C motif chemokine 14 (CXCL14) recruiting macrophages (17) (Figure 1). Of interest is the fact that RAS mutation (G12V) induces ERK5 phosphorylation at Thr 732 (15) akin to the above reported in BRAF melanoma (Figure 1).
NE activated β2-adrenergic receptors in EGFR-mutant NSCLC cells, such as H3255 or HCC827, with 1 or 10 µM NE for 24 hours and IL-6 secretion by enzyme-linked immunosorbent assay (ELISA) was significantly increased. High IL-6 levels in NSCLC patients were also associated with worse progression-frees survival. Smokers have higher concentrations of IL-6 than nonsmokers (10). In addition, IL-6 mRNA expression was increased after NE (10 µM) stimulation for three hours. Propranolol (B-AR inhibitor) blocked NE-induced IL-6. β2-adrenergic receptor activated cAMP signaling pathway through stimulation of adenylyl cyclase (Figure 1). It is plausible that the activation of Gs-coupled receptors by epinephrine stimulation increases LATS1/2 kinase activity, inhibiting YAP function as above commented (8) by phosphorylation of YAP at Ser 127 (18). Forskolin is an activator of adenylyl cyclase that results in increased cAMP production and YAP phosphorylation (8). Forskolin has also been seen to increase IL-6 similar to that induced by NE (10). Moreover, NE induced via PKC LKB1 Ser 248 phosphorylation, a modification that inhibits LKB1 function (10) (Figure 1). Furthermore, phosphorylation of Raf kinase inhibitory protein (RKIP) at Ser 153 by PKC triggers a switch from inhibition of Raf to inhibition of the G protein coupled receptor kinase 2 (GRK2), enhancing signaling by β-adrenergic receptor that activates PKA (19) (Figure 1). It is tempting to posit that the inhibition of GRK2 by RKIP2 can enhance KISS1/GPR54 since GRK2 has been reported to promote KISS1/GPR54 internalization via clathrin-coated pits (6). The new data shows that RKIP phosphorylated at Ser 153 by PKC inhibits GRK2, reducing down-regulation of the β-adrenergic receptor. β-adrenergic receptor, in turn, activates PKA, which then phosphorylates RKIP at Ser 51, leading to increased phosphorylation by PKC at Ser 153 (19) (Figure 1).
Therefore, activation of Gs-coupled receptors by epinephrine could maintain the Hippo pathway and YAP cytoplasmic retention while KPs could activate the YAP pathway via Gαq/11 coupled receptors that inhibit LATS1/2 kinases. The interplay of both neurotransmitters, epinephrine/NE and KPs, warrants further investigation as part of biopsy liquid analytes and investigation in cancer cell lines (Figure 1). The report by Gatti et al. (4) illuminates the need for further integrative studies to decipher the complex interplay between different signaling pathways.
It has recently been seen that a cell surface tumor suppressor, KIRREL 1, is involved in the Hippo pathway binding directly to Salvador 1 (SAV1) upstream of LATS1/2 (20,21) (Figure 1). A previous study using CRISPR screen in 3D cancer model identified KIRREL as a cancer driver in H23 (KRAS-mutant) and H1975 (EGFR-mutant) cells (22).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-494/coif). RR serves as an Editor-in-Chief of Translational Lung Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Joosse SA, Pantel K. Circulating DNA and Liquid Biopsies in the Management of Patients with Cancer. Cancer Res 2022;82:2213-5. [Crossref] [PubMed]
- Guzman S, Brackstone M, Radovick S, et al. KISS1/KISS1R in Cancer: Friend or Foe? Front Endocrinol (Lausanne) 2018;9:437. [Crossref] [PubMed]
- Corno C, Perego P. KiSS1 in regulation of metastasis and response to antitumor drugs. Drug Resist Updat 2019;42:12-21. [Crossref] [PubMed]
- Gatti L, Rolli L, Corno C, et al. Increased serum levels of KiSS1- derived peptides in non-small cell lung cancer patient liquid biopsies and biological relevance. Transl Lung Cancer Res 2022;11:1315-26. [Crossref] [PubMed]
- Stathaki M, Stamatiou ME, Magioris G, et al. The role of kisspeptin system in cancer biology. Crit Rev Oncol Hematol 2019;142:130-40. [Crossref] [PubMed]
- Fratangelo F, Carriero MV, Motti ML. Controversial Role of Kisspeptins/KiSS-1R Signaling System in Tumor Development. Front Endocrinol (Lausanne) 2018;9:192. [Crossref] [PubMed]
- Zhu N, Zhao M, Song Y, et al. The KiSS-1/GPR54 system: Essential roles in physiological homeostasis and cancer biology. Genes Dis 2020;9:28-40. [Crossref] [PubMed]
- Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012;150:780-91. [Crossref] [PubMed]
- Yang H, Xia L, Chen J, et al. Stress-glucocorticoid-TSC22D3 axis compromises therapy-induced antitumor immunity. Nat Med 2019;25:1428-41. [Crossref] [PubMed]
- Nilsson MB, Sun H, Diao L, et al. Stress hormones promote EGFR inhibitor resistance in NSCLC: Implications for combinations with β-blockers. Sci Transl Med 2017;9:eaao4307. [Crossref] [PubMed]
- Zhang S, Yu F, Che A, et al. Neuroendocrine Regulation of Stress-Induced T Cell Dysfunction during Lung Cancer Immunosurveillance via the Kisspeptin/GPR54 Signaling Pathway. Adv Sci (Weinh) 2022;9:e2104132. [Crossref] [PubMed]
- Jiang SH, Hu LP, Wang X, et al. Neurotransmitters: emerging targets in cancer. Oncogene 2020;39:503-15. [Crossref] [PubMed]
- Tusa I, Gagliardi S, Tubita A, et al. ERK5 is activated by oncogenic BRAF and promotes melanoma growth. Oncogene 2018;37:2601-14. [Crossref] [PubMed]
- Stecca B, Rovida E. Impact of ERK5 on the Hallmarks of Cancer. Int J Mol Sci 2019;20:1426. [Crossref] [PubMed]
- Tubita A, Lombardi Z, Tusa I, et al. Beyond Kinase Activity: ERK5 Nucleo-Cytoplasmic Shuttling as a Novel Target for Anticancer Therapy. Int J Mol Sci 2020;21:938. [Crossref] [PubMed]
- Tubita A, Lombardi Z, Tusa I, et al. Inhibition of ERK5 Elicits Cellular Senescence in Melanoma via the Cyclin-Dependent Kinase Inhibitor p21. Cancer Res 2022;82:447-57. [Crossref] [PubMed]
- Sturmlechner I, Zhang C, Sine CC, et al. p21 produces a bioactive secretome that places stressed cells under immunosurveillance. Science 2021;374:eabb3420. [Crossref] [PubMed]
- Chaib I, Karachaliou N, Pilotto S, et al. Co-activation of STAT3 and YES-Associated Protein 1 (YAP1) Pathway in EGFR-Mutant NSCLC. J Natl Cancer Inst 2017; [Crossref] [PubMed]
- Lee J, Olivieri C, Ong C, et al. Raf Kinase Inhibitory Protein regulates the cAMP-dependent protein kinase signaling pathway through a positive feedback loop. Proc Natl Acad Sci U S A 2022;119:e2121867119. [Crossref] [PubMed]
- Wang C, Feng X, Su D, et al. Integrated screens uncover a cell surface tumor suppressor gene KIRREL involved in Hippo pathway. Proc Natl Acad Sci U S A 2022;119:e2121779119. [Crossref] [PubMed]
- Paul A, Annunziato S, Lu B, et al. Cell adhesion molecule KIRREL1 is a feedback regulator of Hippo signaling recruiting SAV1 to cell-cell contact sites. Nat Commun 2022;13:930. [Crossref] [PubMed]
- Han K, Pierce SE, Li A, et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 2020;580:136-41. [Crossref] [PubMed]


