
Clinical use of 18F-FDG PET/CT in the differential diagnosis of patients with primary and secondary adenoid cystic carcinoma of the lung: a retrospective cohort study
Introduction
Adenoid cystic carcinoma (ACC) is a rare malignancy that most frequently originates from the salivary glands of the head and neck (1). It is thought to be an indolent, low-grade tumor, characterized by a slow clinical course, frequent local recurrences, high propensity for perineural invasion, and late distant metastases (1-3). Lung is the most common site of distant metastasis of ACC derived from the head and neck, followed by bone, liver, and brain (4,5). In even rarer cases, ACC may arise from the submucosal tracheobronchial glands in the pulmonary system, and this accounts for a mere 0.04–0.2% of all primary lung tumors (6-8). It is difficult to distinguish between primary ACC of the lung (PACCL) and metastatic ACC of the lung (ACCL), as both conditions manifest atypical respiratory obstructive symptoms, including cough, chest pain, and dyspnea (2,9,10). Currently, morphology differences are the only distinguishing feature between PACCL and secondary ACC of the lung (SACCL).
Due to its rarity, primary or secondary ACCL has only been reported in sporadic cases or small clinical series (9,11,12). The clinical characteristics and imaging features of these two tumors of different origins, as well as the biological differences between them, remain poorly defined. Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has been established as an effective imaging modality, providing both morphological and metabolic information on tumors, which is very useful in distinguishing between malignancies and benign diseases and assessing tumor staging and prognosis (13). For example, glucose metabolism, assessed by 18F-FDG PET/CT, has been reported to be associated with the prognosis of ACC of the head and neck (14,15). Furthermore, previous study has shown that 18F-FDG PET/CT was useful in the differential diagnosis between primary and metastatic lung lesions (16). Discrimination between primary and secondary lung cancer is critical to guide patient management, such as the choice of surgical approach and adjuvant therapy. However, few studies have been reported on the role of 18F-FDG PET/CT in ACCL, separately in PACCL and SACCL (17-19).
Therefore, this current study retrospectively analyzed and compared the clinicopathological characteristics, 18F-FDG PET/CT findings, and outcomes of PACCL and SACCL. In addition, the association between 18F-FDG PET/CT metabolic parameters and clinicopathological characteristics in patients with ACCL was explored. Treatment outcomes and progression-free survival (PFS) of these patients were also evaluated and analyzed. We present the following article in accordance with the STARD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-509/rc).
Methods
Patients
A total of 40 consecutive patients with pathologically confirmed ACCL who underwent 18F-FDG PET/CT examination in the Guangdong Provincial People’s Hospital or the Shanghai Pulmonary Hospital from July 2008 to May 2021 were retrospectively included in this study. The following inclusion criteria were applied: (I) patients underwent tumor resection or puncture biopsy for pathological type confirmation within 2 weeks after the 18F-FDG PET/CT scan; (II) patients could have a history of ACC with confirmed pathological records; and (III) patients had no history of a second pathological type of tumors or other coexisting malignant tumors. The clinical data, including clinical symptoms, laboratory test results, 18F-FDG PET/CT metabolic parameters, and therapeutic strategies of these 40 patients were collected and analyzed. A total of 22 patients underwent surgical tumor resection and their postoperative histopathological results were also examined, including neural invasion, lymphatic metastasis, and extraparenchymal extension. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Guangdong Provincial People’s Hospital (No. KY-Z-2022-267-01) and the Ethics Committee of the Shanghai Pulmonary Hospital (No. K21-143Y). Written informed consent was waived due to the retrospective nature of this investigation.
18F-FDG PET/CT scan
PET/CT examinations were performed using a Biograph 16 HR (Siemens Healthineers, Erlangen, Germany) or a Biograph 64 system (Siemens Healthineers, Erlangen, Germany). All patients were required to fast and avoid strenuous exercise at least 6 hours before 18F-FDG injection, and the level of fasting blood glucose was no more than 11.0 mmol/L. Six or seven-bed positions were imaged from the base of the skull to the mid-thigh at approximately 60±5 minutes after intravenous injection of 3.7–7.4 MBq of 18F-FDG per kilogram of body weight. PET images were acquired for 2–3 minutes per bed position. The ordered-subset expectation maximization algorithm was used for all image reconstructions, incorporating a CT-based transmission map.
PET/CT imaging analysis
PET metabolic parameters were analyzed through syngo station (Siemens Healthineers, Erlangen, Germany). The volume of interest (VOI) was manually drawn on the lesion, or the largest lesion if more than one lesion was detected in the lung. The maximum value of a VOI was defined as the maximum standard uptake value (SUVmax). The metabolic tumor volume (MTV) of the lesion was computed with 40% of SUVmax as the threshold, and the total lesion glycolysis (TLG) of the lesion was calculated according to the following formula: TLG = SUVmean × MTV. The SUVmax ratios of tumor-to-mediastinal blood pool and tumor-to-liver were calculated. PET/CT results were independently analyzed and interpreted by two experienced nuclear medicine physicians who were blinded to the clinical and pathological information of all patients.
Pathology
All 40 patients in this study underwent complete tumor resection or puncture biopsy for pathological type confirmation within 2 weeks after the 18F-FDG PET/CT scan. The specimens were carefully examined, and the section with sufficient malignant cells was subjected to histopathological confirmation. Routine immunohistochemical staining of CD117, P63, S-100, thyroid transcription factor 1 (TTF-1), Ki-67, smooth muscle actin (SMA), glial fibrillary acidic protein (GFAP), calponin, and creatine kinase (CK) was performed for confirmation of ACC (20,21). The histopathological results were reviewed by two experienced pathologists.
Follow-up
Of all enrolled patients, 30 patients underwent post-treatment follow-up, while 10 patients were lost to follow-up. The median duration of follow-up was 25.5 months with a range of 7.7–104.7 months. PFS was defined as the time interval from the pre-treatment PET/CT scan to the date of detecting post-treatment tumor progression or death from any cause. Follow-up was performed using patient medical records and telephone consultation.
Statistical analysis
Data analysis was performed with the Statistical Package for Social Sciences (SPSS) Version 26.0 program (IBM Corporation, Armonk, NY, USA). Continuous variables are presented as median (range), and categorical variables are reported as frequency. Student’s t-tests or Mann-Whitney U tests were used to analyze the differences for continuous variables among different groups. Pearson’s Chi-square tests (χ2 test) or Fisher’s exact tests were used to compare the differences for categorical variables. Spearman’s correlation coefficients were used for correlation analyses. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal SUVmax cut-off value for distinguishing PACCL from SACCL. In this study, An AUC >0.8 with >70% sensitivity and specificity is considered the threshold for a good diagnostic test. Kaplan-Meier survival analyses and log-rank tests were performed to predict PFS and compare difference in PFS of the two groups. The prognostic value of PET/CT metabolic parameters and clinicopathological features was evaluated by univariate Cox regression analysis, and the significant variables (P<0.05) in univariate analysis were further included in multivariate Cox regression analysis. All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Patient characteristics
A total of 40 patients with ACCL were enrolled in this study, including 29 patients with PACCL and 11 patients with SACCL that had metastasized from the ACC of the head and neck (sinonasal cavity/nasopharynx, 4 patients; parotid gland, 2 patients; lacrimal gland, 2 patients; maxillary sinus, 2 patients; and oral cavity, 1 patient). The clinical characteristics of the two groups are shown in Table 1. Patients with PACCL tended to have a higher prevalence of cough as a clinical symptom (37.9% vs. 0.0%), tumor location at the trachea and/or main bronchus (82.8% vs. 18.2%), and less than 3 tumors (93.1% vs. 36.4%) compared with SACCL patients (P<0.05). Meanwhile, the prevalence of subclinical manifestation tended to be higher in patients with SACCL compared to patients with PACCL (27.6% vs. 72.7%). No significant differences in age, gender, other clinical symptoms, tumor size (maximum diameter), and serum tumor markers were found between the two groups (P>0.05).
Table 1
| Characteristics | All | PACCL | SACCL | P value |
|---|---|---|---|---|
| Age (years), median [range] | 49 [21–80] | 50 [21–64] | 46 [30–80] | 0.591 |
| <49, n (%) | 20 (50.0) | 13 (44.8) | 7 (63.6) | 0.480 |
| ≥49, n (%) | 20 (50.0) | 16 (55.2) | 4 (36.4) | |
| Gender, n (%) | ||||
| Male | 18 (45.0) | 14 (48.3) | 4 (36.4) | 0.723 |
| Female | 22 (55.0) | 15 (51.7) | 7 (63.6) | |
| Clinical symptoms | ||||
| Duration (months), median (range) | 6.0 (0.3–24.0) | 6.0 (0.3–24.0) | 6.0 (1.0–6.0) | NA |
| Cough, n (%) | 11 (27.5) | 11 (37.9) | 0 (0.0) | 0.009* |
| Dyspnea, n (%) | 7 (17.5) | 7 (5.5) | 0 (0.0) | 0.159 |
| Chest pain, n (%) | 5 (12.5) | 2 (6.9) | 3 (27.3) | 0.117 |
| Chest congestion, n (%) | 5 (12.5) | 4 (13.8) | 1 (9.1) | 1.000 |
| Blood-streaked sputum, n (%) | 4 (10.0) | 4 (13.8) | 0 (0.0) | 0.293 |
| Hoarse voice, n (%) | 1 (2.5) | 1 (3.4) | 0 (0.0) | 1.000 |
| Subclinical, n (%) | 16 (40.0) | 8 (27.6) | 8 (72.7) | 0.014* |
| Location, n (%) | ||||
| Trachea, main bronchus | 26 (65.0) | 24 (82.8) | 2 (18.2) | <0.001* |
| Trachea only | 18 (18.0) | 16 (55.2) | 2 (18.2) | |
| Main bronchus only | 7 (17.5) | 7 (24.1) | 0 (0.0) | |
| Trachea and main bronchus | 1 (2.5) | 1 (3.4) | 0 (0.0) | |
| Lung, pleura | 14 (35.0) | 5 (17.2) | 9 (81.8) | |
| Lung only | 10 (25.0) | 4 (13.8) | 6 (54.5) | |
| Pleura only | 1 (2.5) | 0 (0.0) | 1 (9.1) | |
| Lung and pleura | 3 (7.5) | 1 (3.4) | 2 (18.2) | |
| Tumor number in lung, n (%) | ||||
| <3 | 31 (77.5) | 27 (93.1) | 4 (36.4) | 0.001* |
| 1 | 29 (72.5) | 26 (89.7) | 3 (27.3) | |
| 2 | 2 (5.0) | 1 (3.4) | 1 (9.1) | |
| ≥3 | 9 (22.5) | 2 (6.9) | 7 (63.6) | |
| Tumor size (mm), median [range] | 24 [9–91] | 24 [11–53] | 23 [9–91] | 0.835 |
| <24, n (%) | 20 (50.0) | 14 (48.3) | 6 (54.5) | 1.000 |
| ≥24, n (%) | 20 (50.0) | 15 (51.7) | 5 (45.5) | |
| Serum tumor marker, n (%) | ||||
| CEA ↑ | 1 (2.5) | 0 (0.0) | 1 (9.1) | 0.275 |
| NSE ↑ | 1 (2.5) | 0 (0.0) | 1 (9.1) | 0.275 |
| CA199 ↑ | 1 (2.5) | 1 (3.4) | 0 (0.0) | 1.000 |
| PET/CT parameters, median (range) | ||||
| SUVmax of tumor | 4.0 (1.0–11.0) | 4.4 (1.6–11.0) | 2.8 (1.0–5.3) | 0.002* |
| MTV of tumor | 6.3 (0.8–144.3) | 8.6 (0.8–74.2) | 3.6 (1.6–2,144.3) | 0.676 |
| TLG of tumor | 18.9 (1.0–397.8) | 24.2 (1.0–363.7) | 6.0 (1.4–397.8) | 0.139 |
| SUVmax of mediastinal blood pool | 2.0 (1.0–3.3) | 1.3 (2.1–3.3) | 1.0 (1.7–3.1) | 0.145 |
| SUVmax of liver | 1.9 (3.1–4.1) | 2.1 (3.1–4.1) | 1.9 (3.1–4.0) | 0.159 |
| T/M | 2.0 (0.7–5.1) | 2.5 (0.8–5.1) | 1.6 (0.7–2.4) | 0.008* |
| T/L | 1.5 (0.4–3.0) | 1.6 (0.6–3.0) | 0.9 (0.4–1.7) | 0.013* |
| Treatment, n (%) | ||||
| Surgery alone | 13 (32.5) | 8 (27.6) | 5 (45.5) | NA |
| Surgery and adjuvant therapy# | 9 (22.5) | 9 (31.0) | 0 (0.0) | NA |
| Other therapy## | 14 (35.0) | 10 (34.5) | 4 (36.4) | NA |
| Untreated | 4 (10.0) | 2 (6.9) | 2 (18.2) | NA |
| Surgical pathology, n (%) | ||||
| Perineural invasion | 6 (28.6) | 5 (31.3) | 1 (20.0) | NA |
| Pleural invasion | 2 (9.5) | 0 (0.0) | 2 (40.0) | NA |
| Lymphatic invasion | 3 (14.3) | 3 (18.8) | 0 (0.0) | NA |
| Extraparenchymal extension | 6 (28.6) | 6 (37.5) | 0 (0.0) | NA |
*, statistically significant; #, adjuvant therapy, including chemotherapy, radiotherapy, and immunotherapy; ##, other therapy, including chemotherapy, radiotherapy, immunotherapy, and targeted therapy. PET/CT, positron emission tomography/computed tomography; ACCL, adenoid cystic carcinoma of the lung; PACCL, primary adenoid cystic carcinoma of the lung; SACCL, secondary adenoid cystic carcinoma of the lung; CEA, carcinoembryonic antigen; NSE, neuron-specific enolase; CA199, carbohydrate antigen 199; T/M, the SUVmax ratio of tumor-to-mediastinal blood pool; T/L, the SUVmax ratio of tumor-to-liver; NA, not applicable; SUVmax, maximum standard uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis.
PET/CT
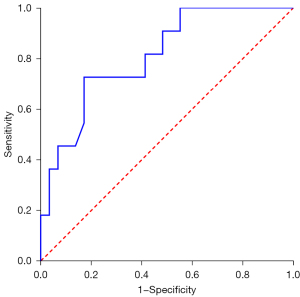
During PET/CT imaging, 18F-FDG uptake was shown in all 40 ACCL cases (the largest lesion was assessed if more than one lesion was detected), with a median SUVmax, MTV, and TLG of 4.0 (range, 1.0–11.0), 6.3 (range, 0.8–144.3), and 18.9 (range, 1.0–397.8), respectively. The SUVmax of PACCL was significantly higher than that of SACCL (P<0.05, Table 1, Figure 1). Furthermore, at ROC analysis, the optimal SUVmax cut-off value for distinguishing PACCL from SACCL was equal to 3.2 with an area under the curve of 0.810 [95% confidence interval (CI): 0.668 to 0.953], sensitivity and specificity of 82.8% and 72.7%, respectively (Figure 2). The SUVmax ratios of tumor-to-mediastinal blood pool and tumor-to-liver were also significantly higher in PACCL compared to those of SACCL (P<0.05). Neither significant differences in MTV, TLG, SUVmax of the mediastinal blood pool, nor SUVmax of the liver were found between the two groups (P>0.05).
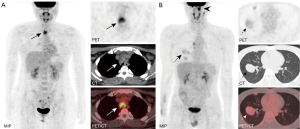
Treatment and surgical pathology
As shown in Table 1, 22 out of the 40 patients underwent surgery for tumor resection, including 9 patients who accepted additional adjuvant therapy. Non-surgical treatment was administered in 14 patients, including chemotherapy, radiotherapy, immunotherapy, and targeted therapy. Four patients did not undergo any treatment. Among the 22 patients undergoing surgical treatment, post-surgical pathology revealed perineural invasion in 6 cases, pleural invasion in 2 cases, lymphatic invasion in 3 cases, and extraparenchymal extension in 6 cases.
PET/CT parameters association with clinical characteristics
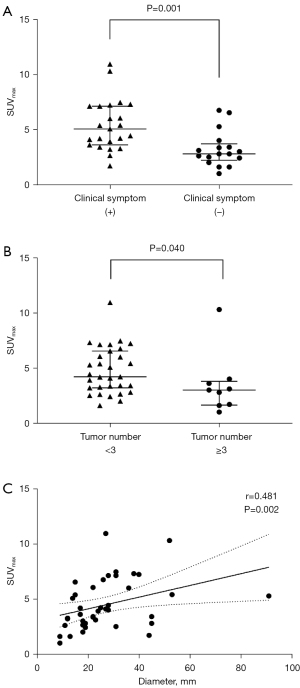
For all 40 patients enrolled, the median SUVmax of ACC in patients with and without clinical symptoms was 4.8 (range, 1.7–11.0) and 2.8 (range, 1.0–6.8), respectively, and a significant difference was found between these two groups (P=0.001, Figure 3A). In addition, the SUVmax in patients with <3 tumors (median, 4.2 tumors; range, 1.6–11.0) was significantly higher than that in patients with ≥3 tumors (median, 3.0 tumors; range, 1.0–10.3) (P=0.040, Figure 3B). Moreover, there was a significant correlation between SUVmax and tumor size (r=0.481, P=0.002, Figure 3C). No significant correlation was observed between SUVmax and other clinicopathological factors, including age, gender, tumor location, serum tumor markers, and post-surgical pathology (P>0.05).
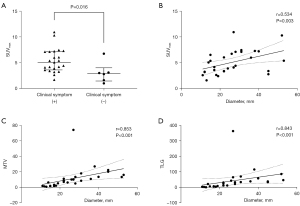
In the PACCL group, the SUVmax of lesions in patients with clinical symptoms (median, 5.4; range, 2.6–11.0) was also significantly higher than that in subclinical patients (median, 3.1; range, 1.6–6.8) (P=0.016, Figure 4A). The SUVmax was significantly associated with tumor size in PACCL patients (r=0.534, P=0.003, Figure 4B). Furthermore, both the MTV (r=0.853, P<0.001, Figure 4C) and TLG (r=0.843, P<0.001, Figure 4D) were significantly correlated with the tumor size of the PACCL. Neither significant differences between PET/CT metabolic parameters nor other clinicopathological features in PACCL were noted (P>0.05).
Follow-up and prognosis
In patients with available data at follow-up (n=30), remission was achieved in 23 patients, while disease progression was observed in 7 patients, including 5 deaths. Specifically, there were 2 cases of disease progression (lung metastasis) and 1 case of death in the PACCL group (n=23), and 4 deaths in the SACCL group (n=7).
Univariate Cox regression analysis showed that tumor origin (SACCL vs. PACCL), tumor location, and tumor number were significantly associated with PFS (P<0.05, Table 2). Multivariate Cox regression analysis adjusted for these relevant factors showed that no variable had any significant association with PFS (P>0.05, Table 2). The median PFS was 71.1 months, and the 12- and 24-month PFS rates were 96.1% and 91.6%, respectively (Figure 5A).
Table 2
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| P value | HR | 95% CI | P value | HR | 95% CI | ||
| Age | 0.435 | 0.97 | 0.90–1.05 | – | – | – | |
| Gender (female) | 0.350 | 0.48 | 0.10–2.22 | – | – | – | |
| Clinical symptoms | 0.582 | 0.66 | 0.15–2.96 | – | – | – | |
| Tumor origin (S vs. P) | 0.039* | 5.03 | 1.09–23.27 | 0.539 | 1.90 | 0.24–14.83 | |
| Tumor location (lung, pleura) | 0.005* | 8.34 | 1.00–69.78 | 0.275 | 4.84 | 0.29–81.79 | |
| Tumor number (≥3) | 0.039* | 5.75 | 1.10–30.16 | 0.865 | 1.25 | 0.09–17.26 | |
| Tumor size | 0.995 | 1.00 | 0.95–1.05 | – | – | – | |
| SUVmax | 0.879 | 0.98 | 0.73–1.31 | – | – | – | |
| MTV | 0.683 | 1.02 | 0.92–1.03 | – | – | – | |
| TLG | 0.855 | 1.00 | 0.97–1.03 | – | – | – | |
| Treatment (no surgery) | 0.803 | 1.22 | 0.26–5.67 | – | – | – | |
*, statistically significant. S and P represents secondary and primary adenoid cystic carcinoma of the lung. PFS, progression-free survival; ACCL, adenoid cystic carcinoma of the lung; SUVmax, maximum standard uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; HR, hazard ratio; CI, confidence interval.
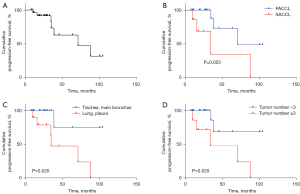
Moreover, Kaplan-Meier survival analysis demonstrated that the PFS rate of patients with SACCL was significantly lower than that of PACCL patients (P<0.05, Figure 5B), and the PFS rate of patients with lesions located at the lung and/or pleura was significantly lower than that of patients with lesions located at the trachea and/or main bronchus (P<0.05, Figure 5C). Furthermore, the PFS rate of patients with ≥3 tumor ACC lesions was significantly lower than that of patients with <3 tumor lesions (P<0.05, Figure 5D).
Univariate Cox regression analysis revealed that age, gender, clinical symptom, tumor location, tumor number, tumor size, PET/CT metabolic parameters, and treatment was not associated with the PFS of PACCL patients (P>0.05, Table 3).
Table 3
| Variables | Univariate analysis | P value | |
|---|---|---|---|
| HR | 95% CI | ||
| Age | 0.98 | 0.88–1.08 | 0.653 |
| Gender (female) | 0.35 | 0.02–6.15 | 0.470 |
| Clinical symptoms | 29.43 | 0.00–71,135,719.67 | 0.652 |
| Tumor location (lung, pleura) | 3.77 | 0.34–41.64 | 0.279 |
| Tumor number (≥3) | 1.98 | 0.17–23.58 | 0.591 |
| Tumor size | 1.00 | 0.92–1.10 | 0.980 |
| SUVmax | 1.30 | 0.83–2.03 | 0.259 |
| MTV | 0.89 | 0.65–1.21 | 0.445 |
| TLG | 1.01 | 0.97–1.05 | 0.720 |
| Treatment (no surgery) | 0.015 | 0.00–1,327.44 | 0.471 |
PFS, progression-free survival; PACCL, primary adenoid cystic carcinoma of the lung; SUVmax, maximum standard uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; HR, hazard ratio; CI, confidence interval.
Discussion
The presence of ACCL is rare, and it either primarily originates from the submucosa glands distributed along in the tracheobronchial system or metastasizes from the ACC of the head and neck. Consistent with previous reports (4,7,22), in our study cohort, PACCL was more often observed in the trachea and major bronchus and rarely in the peripheral lung, while SACCL mainly occurred in the peripheral or segmental localization of the lung. In agreement with the literature (2,9,23), the most common clinical symptoms in our patients with ACCL were cough and dyspnea. Notably, in our study, patients with PACCL manifested a higher incidence of cough compared to SACCL patients, which may be due to the predilection site of PACCL in the trachea and major bronchus. Patients with SACCL were more likely to be asymptomatic and present with multiple lesions compared to PACCL patients, and this may be due to its preferential location in the peripheral lung via hematogenous metastasis from the ACC of the head and neck (24). Moreover, the current data showed that there were no differences in age, sex, tumor size, and serum tumor markers between the PACCL and SACCL groups. Due to nonspecific manifestations, it is challenging to distinguish ACCL from other pulmonary malignancies and benign diseases. Furthermore, it is also difficult to make a differential diagnosis between PACCL and SACCL merely based on their clinical characteristics and morphology. Thus, acquisition of previous tumor history of the patient plays a critical role in their differential diagnosis.
18F-FDG PET/CT has been shown to be useful in differentiating malignant and benign diseases and predicting metastasis and prognosis in ACC of the head and neck (15,18,25). The SUVmax is the most commonly used metabolic parameter of 18F-FDG PET/CT, and reflects the tumor metabolic activity and aggressiveness of the tumor (13). A number of studies have reported on the 18F-FDG PET/CT findings of ACCL, with the SUVmax ranging from 2.5 to 8.8 (11,17,26). All the lesions in our study showed increased FDG uptake to varying degrees, with a median SUVmax of 4.0, indicative of the malignant nature of the tumor. Moreover, the SUVmax of PACCL was significantly higher than that of SACCL, and the optimal differential cut-off value was 3.2, which indicated that PACCL may possess higher tumor aggressiveness compared to SACCL, although the prognosis seems better in the first one as compared to the second one. Probably it would be due to the fact that PACCL is more likely to be surgically resected for its fewer and more confined lesions. In addition, patients with clinical symptoms or those with fewer lesions tended to have higher SUVmax in the whole cohort, which was in line with the greater FDG uptake in PACCL since the presence of clinical symptom and only one or two detected lesions was more frequent in PACCL. Furthermore, a higher SUVmax was also correlated with the presence of clinical symptoms in PACCL patients. This may be explained by the fact that the more aggressive the tumor, the more it invades the surrounding structures and causes clinical symptoms. Moreover, the current study demonstrated that glucose metabolism of the ACCL was associated with the tumor size in both the whole cohort and the PACCL group.
Surgical resection is the first choice of treatment, whenever feasible, for ACCL patients. Multiple studies have shown that surgery can provide a significant survival advantage in PACCL over non-surgical treatment, regardless of complete or incomplete resection (3,22,27-29). Even for SACCL from the head and neck, lung metastasectomy should be considered as a therapeutic option to control disseminated disease and to achieve better survival when complete resection is feasible and the metastasis time interval is greater than 36 months (30). However, in the present study, no significant difference in PFS interval was found between patients who had undergone surgery and those in the non-surgery group. Due to the rarity of ACCL, adjuvant radiotherapy for postoperative ACCL patients remains controversial. Several studies have demonstrated that postoperative radiotherapy significantly improves overall survival, especially in ACCL patients with lymph node metastasis or positive margin resection (R1) (22,31,32). In the current study, the majority of patients (22/40) underwent surgery for tumor resection. All patients had negative margin resection (R0) according to postoperative pathology results. For 5 patients who were confirmed by surgical histopathology to have lymphatic metastasis, perineural invasion, or extraparenchymal extension, postoperative radiotherapy was additionally administered. Regarding advanced inoperable ACCL, palliative chemotherapy and radiotherapy should be considered as an alternative therapy (23,33,34). Wu et al. reported a case with unresectable tracheal ACC who responded rapidly to radiotherapy (35). Moreover, only a few case reports have indicated that ACCL patients may be candidates for target therapy (36). Expression of the epidermal growth factor receptor (EGFR) in the ACC of the lung has been reported, but the frequency of EGFR mutation is rare (37). Mutational landscape analysis conducted by Wang et al. showed a distinctive mutational spectrum of PACCL that has frequent mutations in chromatin remodeling and Notch signaling pathway genes, whereas, there was a lack of mutations in the majority of non-small cell lung cancer driver genes, including EGFR (38). However, in the present study, one patient with PACCL presented with an EGFR mutation and was given EGFR- tyrosine kinase inhibitors (TKI) therapy.
ACCL has a significant tendency toward submucosal extension within the airway with circumferential and infiltrative growth and extraluminal extension (11,39). Consistent with previous reports, the prevalence of lymph node metastasis was relatively low in PACCL patients, ranging from 18.2% to 35.3%, whereas, about 50% of PACCL may eventually have hematogenous metastases (9,28,40). Furthermore, ACC originating from the bronchus was shown to be a risk factor for lymph node metastasis compared with tracheal ACC. In our study, lymphatic metastases were relatively uncommon, occurring in only three cases undergoing surgery, two of which were located in the main bronchus and one in the left upper lobe. None of the SACCL patients who underwent surgery were confirmed to have lymphatic metastases, suggesting that ACC was mainly associated with hematogenous metastasis rather than lymphatic metastasis.
As reported in previous studies, the overall 5- and 10-year survival rates of PACCL are 33–88.7% and 10–45%, respectively (3,27,29). The median PFS of PACC of the tracheobronchial tree was 56.9 months and the 5-year PFS was 48.6% in a report by Huo et al. (20). In our analysis, the median PFS of patients with ACCL was 71.1 months, and the 12- and 24-month PFS rates were 96.1% and 91.6%, respectively. For the five deceased cases, the median interval between treatment and death was 34.5 months (range, 10.1–88.0 months), suggesting its indolent nature and the relatively long-term survival even after distant metastasis. In this study, SACCL located at the peripheral lung and multiple ACC lesions were found to be associated with significantly poorer PFS, which may result from more severe cachexia, faster tumor progression, and lost opportunity for a complete surgical resection. A previous investigation reported that the SUVmax of 18F-FDG PET was associated with distant metastasis in the ACC of the head and neck (41). However, no correlation between SUVmax and PFS was identified in this current study. The lack of any significant impact of FDG uptake on PFS may be due to the indolent nature of ACC with late recurrence or metastasis and the relatively short follow-up period. Moreover, lymph node involvement is suggestive of poorer survival in ACC of the lung (31). However, we did not find this correlation in our data, which may be due to the small number of cases with lymphatic metastasis.
There were several limitations to this study. First, the relatively small number of patients and the retrospective nature of the study decreased the statistical power to analyze the relationship between PET/CT parameters and clinicopathological features or the prognosis of ACCL. Second, since the medical data collected ranged over a 10-year period, some patients were lost at follow-up. In addition, the follow-up period for evaluating the conditions of late recurrence or distant metastasis of ACCL was variable and relatively short in some patients. Furthermore, different therapeutic strategies were chosen depending on the patient’s condition, and thus, it was difficult to analyze the effects of different treatments on prognosis. To date, no uniform grading system for primary tracheal malignancies has been established (23). As a result, this study did not analyze the effects of tumor staging of PACCL on prognosis.
Conclusions
This study retrospectively investigated the clinicopathological characteristics, 18F-FDG PET/CT findings, and prognosis of ACCL. SACCL patients were found to more commonly have multiple ACC lesions in the peripheral lung and to be asymptomatic compared to PACCL patients. 18F-FDG PET/CT was useful in differentiating PACCL from SACCL based on the SUVmax. Higher glucose metabolism assessed by 18F-FDG PET/CT, of ACCL was observed in patients with clinical symptoms, fewer lesions, or larger tumor size. SACCL located at peripheral lung or multiple ACC lesion numbers was associated with poorer PFS. However, PET metabolic parameters and clinicopathological features were not independent predictors of PFS in ACCL patients. A larger multicenter trial is warranted to affirm these conclusions.
Acknowledgments
The authors appreciate the academic support from the AME Oncology Collaborative Group.
Funding: This work was supported by the National Program on Key Basic Research Project (973 Program) (No. 2020YFA0211100), the National Natural Science Foundation of China (Nos. 51922077, 51872205, and 81971645), the Foundation of National Facility for Translational Medicine (Shanghai) (No. TMSK-2020-012), the Young Talents Program, Shanghai Municipal Commission of Health and Family Planning Foundation (No. 2017YQ050), the Guangdong Provincial People’s Hospital (No. KY0120211130), and the Guangdong Provincial Key Laboratory of Artificial Intelligence in Medical Image Analysis and Application (No. 2022B1212010011).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-509/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-509/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-509/coif). LE received consulting fees from Ge Healthcare and Blue Earth. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the Guangdong Provincial People’s Hospital (No. KY-Z-2022-267-01) and the Ethics Committee of the Shanghai Pulmonary Hospital (No. K21-143Y). Written informed consent was waived due to the retrospective nature of this investigation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck--An update. Oral Oncol 2015;51:652-61. [Crossref] [PubMed]
- Falk N, Weissferdt A, Kalhor N, et al. Primary Pulmonary Salivary Gland-type Tumors: A Review and Update. Adv Anat Pathol 2016;23:13-23. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96; discussion 1896-7. [Crossref] [PubMed]
- Gao M, Hao Y, Huang MX, et al. Clinicopathological study of distant metastases of salivary adenoid cystic carcinoma. Int J Oral Maxillofac Surg 2013;42:923-8. [Crossref] [PubMed]
- van der Wal JE, Becking AG, Snow GB, et al. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck 2002;24:779-83. [Crossref] [PubMed]
- Gaissert HA, Mark EJ. Tracheobronchial gland tumors. Cancer Control 2006;13:286-94. [Crossref] [PubMed]
- Kawashima O, Hirai T, Kamiyoshihara M, et al. Primary adenoid cystic carcinoma in the lung: report of two cases and therapeutic considerations. Lung Cancer 1998;19:211-7. [Crossref] [PubMed]
- Ohta K, Matsuda S, Okada A, et al. Adenoid cystic carcinoma of the sublingual gland developing lung metastasis 20 years after primary treatment: A case report and literature review. Medicine (Baltimore) 2021;100:e28098. [Crossref] [PubMed]
- Moran CA, Suster S, Koss MN. Primary adenoid cystic carcinoma of the lung. A clinicopathologic and immunohistochemical study of 16 cases. Cancer 1994;73:1390-7. [Crossref] [PubMed]
- Madariaga MLL, Gaissert HA. Overview of malignant tracheal tumors. Ann Cardiothorac Surg 2018;7:244-54. [Crossref] [PubMed]
- Jeong SY, Lee KS, Han J, et al. Integrated PET/CT of salivary gland type carcinoma of the lung in 12 patients. AJR Am J Roentgenol 2007;189:1407-13. [Crossref] [PubMed]
- Li W, Hua W, Yan FG, et al. Adenoid cystic carcinoma of trachea: a case report and review of literature. Chin Med J (Engl) 2012;125:2238-9. [PubMed]
- Beyer T, Townsend DW, Brun T, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000;41:1369-79. [PubMed]
- Jeong HS, Chung MK, Son YI, et al. Role of 18F-FDG PET/CT in management of high-grade salivary gland malignancies. J Nucl Med 2007;48:1237-44. [Crossref] [PubMed]
- Jung JH, Lee SW, Son SH, et al. Clinical impact of 18 F-FDG positron emission tomography/CT on adenoid cystic carcinoma of the head and neck. Head Neck 2017;39:447-55. [Crossref] [PubMed]
- Kirienko M, Cozzi L, Rossi A, et al. Ability of FDG PET and CT radiomics features to differentiate between primary and metastatic lung lesions. Eur J Nucl Med Mol Imaging 2018;45:1649-60. [Crossref] [PubMed]
- Wang SY, Wang SX, Liao JQ, et al. 18F-FDG PET/CT and Contrast-Enhanced CT of Primary Malignant Tracheal Tumor. Clin Nucl Med 2016;41:595-605. [Crossref] [PubMed]
- Park MJ, Oh JS, Roh JL, et al. 18F-FDG PET/CT Versus Contrast-Enhanced CT for Staging and Prognostic Prediction in Patients With Salivary Gland Carcinomas. Clin Nucl Med 2017;42:e149-56. [Crossref] [PubMed]
- Haresh KP, Prabhakar R, Rath GK, et al. Adenoid cystic carcinoma of the trachea treated with PET-CT based intensity modulated radiotherapy. J Thorac Oncol 2008;3:793-5. [Crossref] [PubMed]
- Huo Z, Meng Y, Wu H, et al. Adenoid cystic carcinoma of the tracheobronchial tree: clinicopathologic and immunohistochemical studies of 21 cases. Int J Clin Exp Pathol 2014;7:7527-35. [PubMed]
- Batsakis JG, Luna MA, el-Naggar A. Histopathologic grading of salivary gland neoplasms: III. Adenoid cystic carcinomas. Ann Otol Rhinol Laryngol 1990;99:1007-9. [Crossref] [PubMed]
- Hu MM, Hu Y, He JB, et al. Primary adenoid cystic carcinoma of the lung: Clinicopathological features, treatment and results. Oncol Lett 2015;9:1475-81. [Crossref] [PubMed]
- Zhu F, Liu Z, Hou Y, et al. Primary salivary gland-type lung cancer: clinicopathological analysis of 88 cases from China. J Thorac Oncol 2013;8:1578-84. [Crossref] [PubMed]
- Maziak DE, Todd TR, Keshavjee SH, et al. Adenoid cystic carcinoma of the airway: thirty-two-year experience. J Thorac Cardiovasc Surg 1996;112:1522-31; discussion 1531-2. [Crossref] [PubMed]
- Lim WS, Oh JS, Roh JL, et al. Prediction of distant metastasis and survival in adenoid cystic carcinoma using quantitative 18F-FDG PET/CT measurements. Oral Oncol 2018;77:98-104. [Crossref] [PubMed]
- Han X, Zhang J, Fan J, et al. Radiological and Clinical Features and Outcomes of Patients with Primary Pulmonary Salivary Gland-Type Tumors. Can Respir J 2019;2019:1475024. [Crossref] [PubMed]
- Yang H, Yao F, Tantai J, et al. Resected Tracheal Adenoid Cystic Carcinoma: Improvements in Outcome at a Single Institution. Ann Thorac Surg 2016;101:294-300. [Crossref] [PubMed]
- Kang DY, Yoon YS, Kim HK, et al. Primary salivary gland-type lung cancer: surgical outcomes. Lung Cancer 2011;72:250-4. [Crossref] [PubMed]
- Molina JR, Aubry MC, Lewis JE, et al. Primary salivary gland-type lung cancer: spectrum of clinical presentation, histopathologic and prognostic factors. Cancer 2007;110:2253-9. [Crossref] [PubMed]
- Girelli L, Locati L, Galeone C, et al. Lung metastasectomy in adenoid cystic cancer: Is it worth it? Oral Oncol 2017;65:114-8. [Crossref] [PubMed]
- Wo Y, Li S, Wang Y, et al. Predictors of nodal metastasis and prognostic significance of lymph node ratio and total lymph node count in tracheobronchial adenoid cystic carcinoma. Cancer Manag Res 2018;10:5919-25. [Crossref] [PubMed]
- Zhao L, Zhao Y, Guo JD, et al. Effective Radiotherapy in Tracheobronchial Adenoid Cystic Carcinoma With Positive Surgical Margin. Ann Thorac Surg 2021;112:1585-92. [Crossref] [PubMed]
- Suemitsu R, Okamoto T, Maruyama R, et al. A long-term survivor after aggressive treatment for tracheal adenoid cystic carcinoma: a case report. Ann Thorac Cardiovasc Surg 2007;13:335-7. [PubMed]
- Laurie SA, Ho AL, Fury MG, et al. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol 2011;12:815-24. [Crossref] [PubMed]
- Wu Q, Xu F. Rapid response to radiotherapy in unresectable tracheal adenoid cystic carcinoma: A case report. World J Clin Cases 2021;9:9535-41. [Crossref] [PubMed]
- Song Z, Wu W, Zhang Y. Effective treatment with icotinib in primary adenoid cystic carcinoma of the lung with liver metastasis. J Thorac Oncol 2014;9:e67-9. [Crossref] [PubMed]
- Dahse R, Kosmehl H. Detection of drug-sensitizing EGFR exon 19 deletion mutations in salivary gland carcinoma. Br J Cancer 2008;99:90-2. [Crossref] [PubMed]
- Wang F, Xi SY, Hao WW, et al. Mutational landscape of primary pulmonary salivary gland-type tumors through targeted next-generation sequencing. Lung Cancer 2021;160:1-7. [Crossref] [PubMed]
- Kwak SH, Lee KS, Chung MJ, et al. Adenoid cystic carcinoma of the airways: helical CT and histopathologic correlation. AJR Am J Roentgenol 2004;183:277-81. [Crossref] [PubMed]
- Azar T, Abdul-Karim FW, Tucker HM. Adenoid cystic carcinoma of the trachea. Laryngoscope 1998;108:1297-300. [Crossref] [PubMed]
- Gencturk M, Ozturk K, Koksel Y, et al. Pretreatment quantitative 18F-FDG PET/CT parameters as a predictor of survival in adenoid cystic carcinoma of the salivary glands. Clin Imaging 2019;53:17-24. [Crossref] [PubMed]


