MYC amplification-conferred primary resistance to capmatinib in a MET-amplified NSCLC patient: a case report
Introduction
The treatment of advanced non-small cell lung cancer (NSCLC) has been revolutionized by selectively targeted agents that match driver oncogenic alteration. Genetic alterations that lead to dysregulation of the mesenchymal-epithelial transition factor (MET) have been identified as primary oncogenic drivers in NSCLC, therefore MET regulation presents a promising therapeutic target for advanced NSCLC (1-3). Recently, capmatinib, a potent and selective MET inhibitor, has been reported to elicit good responses in NSCLC with MET exon 14 skipping mutation (METex14), or MET amplification defined by a gene copy number (GCN) of 10 or higher (1,3). The responses were generally higher when capmatinib was administered as the first-line therapy, with an overall response rate of 68% for METex14 and 40% for MET GCN ≥10 (1). Based on these data, capmatinib was granted accelerated approval by the Food and Drug Administration (FDA) for NSCLC with METex14 (4). Despite these promising results, there exists a lack of knowledge, especially in terms of the molecular mechanisms that cause primary resistance to MET inhibitors. Here, we report a case of metastatic NSCLC with MET amplification (GCN ≥10) who showed primary resistance to the first-line use of capmatinib, and refractory to subsequent chemotherapies. Targeted sequencing of the re-biopsied tumor tissue revealed MYC amplification, and patient-derived tumor cells were successfully inhibited with a MYC inhibitor in vitro. These results suggest that MYC amplification could be a potential mechanism for resistance to MET inhibition in NSCLC. We present the following case in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-176/rc).
Case presentation
An 82-year-old male patient was referred to our institute with a right upper lobe (RUL) lung nodule. He had a smoking history of 60 pack-years, and presented with underlying hypertension, diabetes, and rheumatoid arthritis. Initial staging evaluation with chest computed tomography (CT) revealed an RUL mass which was confirmed as adenocarcinoma with percutaneous biopsy. Molecular analyses showed no epidermal growth factor receptor (EGFR) mutation, negative immunostaining results for ALK, but intense staining for MET (intensity 3+/3, and proportion of positive cells >50%). The clinical stage was T1bN0M0, and the patient was considered a candidate for surgical resection. Nevertheless, he was reluctant to undergo surgery due to the potentially high risks associated with old age and underlying medical conditions. Alternatively, radiation therapy was recommended, but he declined and decided to undergo surveillance. However, three months later, CT scans revealed enlargement of the RUL tumor and a new lesion in the liver.
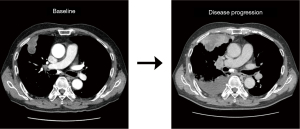
As the initial tumor tissue was intensely stained for MET, he was screened for a phase 2 trial of capmatinib (GEOMETRY mono-1, NCT02414139) as first-line treatment. Real-time polymerase chain reaction (RT-PCR) analysis for METex14 was negative, but MET GCN as determined by fluorescence in situ hybridization (FISH) was 13.5 indicating gene amplification. In addition, immunohistochemistry revealed MET overexpression (3+/3, >50%). Thus, he was enrolled in the trial and received capmatinib (400 mg, twice daily) as the first-line therapy. However, his disease progressed during capmatinib treatment. At the first response evaluation, which was performed after the initial 6 weeks of treatment, CT scans showed systemic progression of the disease including new metastatic lesions (Figure 1). We performed fluorescent in situ hybridization (FISH) for MET, and an amplicon-based next-generation sequencing (NGS, Illumina), with a panel consisting of 91 genes using the re-biopsied tumor samples from the liver. The NGS analysis revealed gene amplification of MYC (estimated copy number 5) and CEBPA without other nucleotide variants or fusion mutations, as well as MET amplification on FISH.
Subsequently, he received paclitaxel and carboplatin as second-line therapy and irinotecan and cisplatin as third-line therapy, without tumor response to either regimen. Multiple brain metastases developed during third-line therapy, and the patient underwent stereotactic radiosurgery but eventually succumbed to death due to disease progression 6 months after the initial diagnosis of metastatic NSCLC.
Following capmatinib treatment, patient-derived cells (PDCs) were cultured in vitro to determine the resistance mechanisms and identify novel treatment strategies. We performed drug susceptibility tests for cytotoxic agents (gemcitabine, cisplatin, paclitaxel, and SN-38), targeted agents (crizotinib, dacomitinib, afatinib, and capmatinib), and investigational agents (OTX-015, BET bromodomain inhibitor; ICX-101, MYC inhibitor). In concordance with MYC amplification shown by NGS, PDCs showed marked overexpression of MYC mRNA (Figure 2A). In the drug susceptibility assay, the viability of PDCs was not suppressed with capmatinib with doses ranging from 0 - 10 µM, which reflected in vivo drug resistance. Notably, the response to ICX-101 (Incurix, Goyang, Korea), an investigational MYC inhibitor (Figures S1,S2), was surprisingly high with an IC50 value of 0.74 µM (Figure 2B). So far, we tested the sensitivity of ICX-101 using multiple NSCLC PDC samples developed in our institute (n=100) and the median IC50 value was 0.91 µM (interquartile range, 0.54–1.54 µM) for all NSCLC PDCs. These data suggest that the case reported here is sensitive to ICX-101, a MYC inhibitor (Figure 2C). In order to see if there is a synergistic effect between ICX-101 and capmatinib, we treated the PDCs of the case patient with both drugs either in combination or as sequential treatments. Co-treatment of ICX-101 and capmatinib was not synergistic, and ICX-101 followed by capmatinib showed only an additive antitumor effect (Figures S3,S4A). However, a synergistic effect was shown when capmatinib was treated with subsequent ICX-101 (Figure S4B). These clinical and in vitro data imply that the primary resistance to capmatinib in this patient even with MET amplification may be conferred by the concurrent MYC amplification, and a MYC inhibitor could be utilized to inhibit resistance to capmatinib.

All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
MET inhibitors have recently been demonstrated as promising novel therapeutic agents for NSCLC with MET aberrations (1,2). Although the clinical benefit of MET inhibitors in these tumors has been shown, questions remain regarding the molecular mechanisms that drive primary or acquired resistance. Studies that investigated the resistance mechanisms against various MET inhibitors in NSCLC with METex14 have shown that alterations in mitogen-activated protein kinase (MAPK) pathway genes (EGFR, KRAS, HER3, and BRAF), de novo mutations of MET, and phosphoinositide-3 kinase (PI3K) signaling alterations were highly implicated (5-8) (Table 1). For MET-amplified lung cancer, a preclinical study demonstrated the importance of EGFR and PI3K signaling in conferring resistance to capmatinib in an NSCLC cell line (9). Notably, another preclinical study suggested the role of MYC as a downstream effector to dictate the therapeutic response of MET inhibitors, and also a driver that leads to both primary and acquired resistance in MET-amplified cancer cells (10). In this study, a lung cancer cell line with MET amplification showed high sensitivity to an investigational MET inhibitor. After generating acquired resistance to this drug, these cells still showed a similar level of MET amplification but an enhanced expression of MYC. They also demonstrated that overexpression of MYC in the parental cell line resulted in diminished drug sensitivity to the MET inhibitor. Together these results suggest that MYC confers both primary and acquired resistance to MET inhibitors, suggesting its role as an essential mediator of therapy resistance (10).
Table 1
| Gene alteration | Method of detection | Reference |
|---|---|---|
| On-target mechanisms | ||
| MET mutation | ||
| H1094Y | NGS (Post-treatment, Tissue/Plasma) | (5) |
| L1195V | NGS (Post-treatment, Tissue/Plasma) | (5) |
| G1163R | NGS (Post-treatment, Tissue/Plasma) | (5) |
| D1228X (H/N/Y) | NGS (Post-treatment, Tissue/Plasma) | (5-7) |
| Y1230X (C/H/S) | NGS (Post-treatment, Tissue/Plasma) | (5,7) |
| MET amplification | NGS (Post-treatment, Tissue) | (5) |
| HGF amplification | NGS (Post-treatment, Tissue) | (6) |
| Off-target mechanisms | ||
| KRAS mutation | ||
| G12X(D/S) | NGS (Post-treatment, Tissue/Plasma) | (5,6) |
| G13V | NGS (Post-treatment, Plasma) | (6) |
| G60D | NGS (Post-treatment, Tissue/Plasma) | (5) |
| V14I | NGS (Post-treatment, Plasma) | (7) |
| KRAS amplification | NGS (Post-treatment, Tissue) | (5,6) |
| EGFR amplification | NGS (Post-treatment, Tissue) | (5,6) |
| BRAF mutation (V600E) | NGS (Post-treatment, Plasma) | (7) |
| BRAF amplification | NGS (Post-treatment, Tissue/Plasma) | (5,7) |
| ERBB3 amplification | NGS (Post-treatment, Tissue/Plasma) | (5,7) |
| PI3KCA mutation | NGS (Pre-treatment) | (8) |
| PTEN Loss of expression | IHC (Pre-treatment) | (8) |
NGS, next generation sequencing; IHC, immunohistochemistry.
MYC deregulation is a well-known oncogenic alteration that is pervasive in many solid tumors and has also been reported to cause resistance to many anti-cancer agents (11). In accordance with this notion, MYC amplification was suggested as a potential cause of primary resistance to crizotinib in a case of advanced NSCLC with ALK-rearrangement (12). However, the prevalence of MYC alteration in MET-driven lung cancer is largely unknown, and the clinical importance of MYC in conferring resistance to MET inhibitors has yet to be validated. Nevertheless, a report showed that concurrent MYC amplification is more common in tumors with MET amplification than in METex14, implying that MYC activation might be more important in MET amplified NSCLC (13).
The MYC family has three paralogs: C-MYC (encoded by MYC), N-MYC (encoded by MYCN), and L-MYC (encoded by MYCL). MYC is enhanced and deregulated in many types of malignancies, which is one of the most frequently deregulated driver genes in human cancer. However, despite its critical role, MYC has been traditionally regarded as an undruggable target because it lacks a preferred pocket to allow high-affinity binding by small molecule drugs (14).
ICX-101 (United States Patent US9951021B2) is an investigational MYC inhibitor developed by Incurix (Goyang, Korea). It is a potent and selective small molecule inhibitor that directly inhibits the binding of MYC/MAX dimers to the DNA binding sequence E-box (Supplementary Data). ICX-101 not only showed very high selectivity for the function of MYC in protein-based assays and cellular-level transcriptome analysis but was also shown to be particularly effective in KRAS mutant NSCLC and small cell lung cancer (SCLC) PDCs compared to other drugs evaluated in the aforementioned PDC screening. Indeed, daily oral administration of ICX-101 in KRAS-mutant NSCLC animal models was found to effectively inhibit cell growth, leading to tumor regression.
As in this case, concurrent MYC amplification could potentially confer primary resistance to capmatinib in NSCLC with MET GCN ≥10 and MET overexpression. Further clinical studies are needed to corroborate these findings, and treatment with MYC inhibitors could be considered as an alternative therapeutic strategy for this subset of patients.
Acknowledgments
Funding: This study was supported by the National Cancer Center Research Grant (grant number 1910281).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-176/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-176/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-176/coif). KCJ has equity in Incurix Co., Ltd. and serves as the president of Incurix Co., Ltd. JYH has received honoraria from AstraZeneca, BMS, F. Hoffmann-La Roche Ltd., MSD, and Takeda; has acted as a consultant or advisor for AstraZeneca, BMS, Eli Lilly, MSD, Novartis, Pfizer, Takeda, Medpacto, Abion, and ONO Pharmaceutical; and has received research funding from F. Hoffmann-La Roche Ltd., ONO Pharmaceutical, Pfizer, and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wolf J, Seto T, Han JY, et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:944-57. [Crossref] [PubMed]
- Paik PK, Felip E, Veillon R, et al. Tepotinib in Non-Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N Engl J Med 2020;383:931-43. [Crossref] [PubMed]
- Choi W, Park SY, Lee Y, et al. The Clinical Impact of Capmatinib in the Treatment of Advanced Non-Small Cell Lung Cancer with MET Exon 14 Skipping Mutation or Gene Amplification. Cancer Res Treat 2021;53:1024-32. [Crossref] [PubMed]
- Mathieu LN, Larkins E, Akinboro O, et al. FDA Approval Summary: Capmatinib and Tepotinib for the Treatment of Metastatic NSCLC Harboring MET Exon 14 Skipping Mutations or Alterations. Clin Cancer Res 2022;28:249-54. [Crossref] [PubMed]
- Recondo G, Bahcall M, Spurr LF, et al. Molecular Mechanisms of Acquired Resistance to MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14-Mutant NSCLC. Clin Cancer Res 2020;26:2615-25. [Crossref] [PubMed]
- Guo R, Offin M, Brannon AR, et al. MET Exon 14-altered Lung Cancers and MET Inhibitor Resistance. Clin Cancer Res 2021;27:799-806. [Crossref] [PubMed]
- Dagogo-Jack I, Moonsamy P, Gainor JF, et al. A Phase 2 Study of Capmatinib in Patients With MET-Altered Lung Cancer Previously Treated With a MET Inhibitor. J Thorac Oncol 2021;16:850-9. [Crossref] [PubMed]
- Jamme P, Fernandes M, Copin MC, et al. Alterations in the PI3K Pathway Drive Resistance to MET Inhibitors in NSCLC Harboring MET Exon 14 Skipping Mutations. J Thorac Oncol 2020;15:741-51. [Crossref] [PubMed]
- Kim S, Kim TM, Kim DW, et al. Acquired Resistance of MET-Amplified Non-small Cell Lung Cancer Cells to the MET Inhibitor Capmatinib. Cancer Res Treat 2019;51:951-62. [Crossref] [PubMed]
- Shen A, Wang L, Huang M, et al. c-Myc alterations confer therapeutic response and acquired resistance to c-Met inhibitors in MET-addicted cancers. Cancer Res 2015;75:4548-59. [Crossref] [PubMed]
- Duffy MJ, O'Grady S, Tang M, et al. MYC as a target for cancer treatment. Cancer Treat Rev 2021;94:102154. [Crossref] [PubMed]
- Rihawi K, Alfieri R, Fiorentino M, et al. MYC Amplification as a Potential Mechanism of Primary Resistance to Crizotinib in ALK-Rearranged Non-Small Cell Lung Cancer: A Brief Report. Transl Oncol 2019;12:116-21. [Crossref] [PubMed]
- Castiglione R, Alidousty C, Holz B, et al. Comparison of the genomic background of MET-altered carcinomas of the lung: biological differences and analogies. Mod Pathol 2019;32:627-38. [Crossref] [PubMed]
- Wang C, Zhang J, Yin J, et al. Alternative approaches to target Myc for cancer treatment. Signal Transduct Target Ther 2021;6:117. [Crossref] [PubMed]