
Cellular senescence and lung cancer prognosis
Pathways responsible for triggering cellular senescence
In addition to limiting the unregulated proliferation of cells, cellular senescence is also a major factor in tumor suppression as well as aging. Among the diverse types of cellular senescence, we can distinguish replicative, which is mediated by the shortening of telomeres, from stress-induced premature senescence, which does not involve telomere shortening. Even though many factors can lead to cellular senescence before cells get old—“premature senescence”, DNA (deoxyribonucleic acid) damage response (DDR) signaling defects are considered one of the common causes of cellular senescence phenotype induction and maintenance (1). Telomere dysfunction often activates DDR signaling, initiating chromosome fusions, and causing breakage-bridge fusion cycles during subsequent progression through the cell cycle, resulting in genome instability and cellular senescence (2). In addition, therapy-induced senescence occurs when cancer cells undergo senescence after being treated with certain chemotherapy agents, and ionizing radiation (3), via the induction of irreparable DNA lesions mediating persistent DDR signaling (4) and innate immune responses (5-7) (Figure 1).
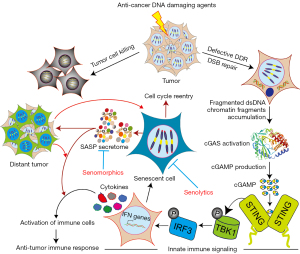
The innate immune responses are triggered in response to DNA damage caused by anti-cancer DNA damaging agents through cytosolic DNA sensors, which sense double-strand DNA in the cytosol. As one of the most important DNA sensors, cyclic GMP-AMP synthase (cGAS) detects cytosolic DNA and activates defense responses by triggering the expression of inflammatory genes. It has been found that tumor-derived DNA, such as DNA of dead tumor cells and free telomeric DNA, along with downregulation of cytoplasmic DNAses like DNAse 2 and TREX1 can be able to trigger cGAS pathway activity (8). Moreover, as the cell cycle progresses through mitosis, irreparable DNA DSBs (double-strand breaks) induced by anti-cancer agents promote the formation of micronuclei, which are cytoplasmic aggregates of damaged genomic DNA encapsulated in a defective nuclear envelope. The disruption of the micronuclear envelope enables DNA to be exposed to the cytosol, culminating in the activation of cGAS. cGAS forms a cyclic di-nucleotide second messenger called 2'-3'-cGAMP which binds and activates the stimulator of interferon genes (STING). Consequently, a series of events occur in these cells, including TANK binding kinase 1 (TBK1) recruitment, interferon regulatory factor 3 (IRF3) activation, innate immune response genes, such as type I interferon cytokines (IFN-I), transactivation (9). Cellular senescence and the production of senescence-associated secretory phenotype (SASP) factors by these senescent cells, in addition to innate immune signaling and autophagy, are some of the outcomes associated with cGAS activation in response to cytosolic DNA accumulation.
Mechanisms of cellular senescence
Similar to multiple pathways responsible for triggering cellular senescence, the process of senescence itself is dynamic and multi-step, with senescent cells constantly evolving and diversifying in a context-dependent manner (10). It is accompanied by multiple cellular and molecular alterations as well as distinct phenotypic changes, including an irreversible arrest in proliferation that is not responsive to mitogens (10). Senescing cells undergo extensive gene expression alterations concomitant with chromatin remodeling and persistent DDR activation (10,11). Senescent cells undergo both morphological and structural changes and develop a complex secretory phenotype called SASP (10,12). Various aspects of senescence are implemented by these complex changes to the cell, such as growth arrest and the expression of SASP secretome components, including chemokines, proinflammatory cytokines, matrix metalloproteinases, noncoding nucleotides (miRNAs, mitochondrial DNA), growth factors, bioactive lipids and vesicles (13). As senescent cells alter their metabolic activity, they are still viable and are resistant to cell death (14). However, the secretome released by the senescent cells are highly immunogenic and are capable of not only promoting sustained recruitment of immune cells to their location (and to the tumor microenvironment) but also actively promoting an innate and adaptive immune response against tumors (15). As a result, senescent cells are cleared by the leukocytes belonging to the innate immune system in a context-dependent manner (16,17). Paradoxically, the SASP secretome may indirectly promote cancer progression by enhancing pre-neoplastic cell growth and altering the tumor microenvironment (13).
Non-small cell lung cancer (NSCLC)—the significance of cellular senescence assessment
In the current report, Domen and colleagues (18) studied cellular senescence in resected NSCLC and its implications for prognosis, according to the definition of a prognostic biomarker, i.e., a prognostic biomarker informs about a cancer outcome (e.g., disease recurrence, disease progression, and death) independent of treatment received (19). Due to the lack of a single, universal, robust biomarker, identifying senescent cells with high sensitivity and specificity, and differentiating them from terminally differentiated, quiescent, and other cells is difficult (10). In addition, there is currently no specific and universal gene set or single protein marker for the detection of cellular senescence (3). Because senescence is heterogeneous and dynamic, a combination of multiple biomarkers is recommended for the correct validation of senescence in cultured cells and in vivo (20). Per the cellular senescence definition, the study by Domen (18) combined the expression of p16, p21, and Ki67, and the accumulation of lipofuscin, as immunohistochemical senescence markers to assess cellular senescence in 155 clinically annotated primary NSCLC tumor specimens. These samples were collected from NSCLC patients with stage I–IV postsurgical pathological tumor-node-metastases (pTNM), who underwent surgery at a single university hospital, treated with or without stage-dependent neoadjuvant therapy.
First, the authors used four different criteria i.e., high-level lipofuscin and low Ki67 expression in combination with either high p16 and/or p21 expression as senescence signatures (SS) for assessing NSCLC specimens using immunohistochemistry. The authors observed that p16INK4a expression positively correlated with high-level lipofuscin accumulation and inversely correlated with p21WAF1/Cip1 expression, in 48 patients of 155 patient samples analyzed. However, no tumoral SS was detected in the remaining 107 patients. Thus, not all NSCLCs harbor well-defined tumoral SS.
Second, the authors also found that the tumoral SS did not differ by age, gender, smoking history (clinical parameters), or by differentiation grade, tumor size and pTNM (pathological parameters), or by EGFR (epidermal growth factor receptor) and KRAS (kirsten rat sarcoma viral oncogene homolog) mutation status. However, with limited oncogenotyping, they found more SS in p53 wild-type tumors (50% of tumors with SS had p53 wild-type). Interestingly, although tumoral SS did not correlate with the status of neoadjuvant therapy, the mean lipofuscin accumulation, and the extent of p16 expression were significantly elevated in samples derived from patients who received neo-adjuvant therapy as compared with patients without neoadjuvant therapy. Tumoral SS was more prevalent in adenocarcinoma samples than other histological subtypes among the patients who received neoadjuvant therapy (n=39). Collectively, more p53 wild-type tumors harbor SS and neoadjuvant therapy can either trigger new SS or reinforce pre-existing SS in NSCLC.
Third, the authors examined the association between SS and overall survival (OS) in 139 histologically heterogeneous groups of patients who underwent surgery with curative intent, excluding patients with pTNM stage IV and resections with microscopically residual tumors. After a median follow-up of 53 months, Kaplan-Meier (KM) survival analysis showed a worse OS in patients with tumoral SS than in patients without tumoral SS, i.e., 62 and 88 months, respectively. Interestingly, among one hundred patients with pTNM stages I–III with no adjuvant systemic therapy or radiotherapy and with a follow-up of 57 months (about 5 years), patients with an SS had significantly shorter median OS than patients without an SS (53 versus 141 months). As a result, the 5-year OS rate was 45% versus 67%, respectively. Additionally, KM analysis of disease-free survival (DFS), also showed that patients with an SS had a lower median DFS than those without an SS (45 versus 55 months). A further evaluation of OS based on high-level lipofuscin and low Ki67 expression with high p16, high p21, or both high p16 and p21, revealed that higher p16 (53 versus 141 months), high p21 (115 Versus 141 months) and high p16 plus p21 (44 versus 141 months) resulted in a worse outcome. As KM survival analysis does not correct for age, pTNM stage I–II, and adjuvant therapy, the authors used a Cox proportional hazards model analysis. By this approach, the authors observed that only the presence of high p16 plus p21 expression but not expression levels of p16 or p21 alone remained as a significant prognostic factor. These analyses show that tumor-associated SSs, characterized by high p16INK4A expression, adversely affects the OS of patients with NSCLC adenocarcinoma.
Lastly, the authors appropriately highlight limitations of their study, such as (I) use of a small population of NSCLC patients (n=155) and (II) results presented in their study were based on a retrospective analysis of a cohort of patients evaluated at a single university hospital for 14 years. They also discuss the role of senescent cells which initially could act as a tumor suppression mechanism, but then by providing secreted factors and other mechanisms to help tumor cells with more malignant potential or immune suppression to arise. Because CDKN2A (p16INK4a), CDKN1A (p21WAF1/Cip1), and MKI67 (Ki67) are not exclusively related to cellular senescence but are involved in a myriad of cellular processes, the immunohistochemical approach used in this study is more reliable to determine senescence than the determination of senescence by mRNA expression. Importantly, the authors raise the possibility of “senolytic therapy” for eliminating senescent cells in NSCLC adenocarcinoma with another possibility being “senomorphic” therapy targeting the SASP secretome. Collectively, this study makes a significant contribution to our limited knowledge of cellular senescence in cancer prognosis, specifically, NSCLC adenocarcinoma.
Clinical translational implications of cellular senescence in lung cancer—developing information on induction of cellular senescence by specific treatments, tumor lineages, and tumor oncogenotypes
The secretome produced by the senescent tumor cells in response to DNA damaging anti-cancer therapies can have two major opposing consequences (Figure 1). One possibility is that senescent cells will stop tumor cell division thus initially attenuating cancer growth. In addition to directly affecting tumor cells and adjacent stromal fibroblasts, secretomes produced by the senescent tumor cells can induce senescence of nearby tumor cells through a paracrine mechanism, resulting in cancer cell elimination via the recruitment of immune cells (21). As opposed to this, senescent cells can contribute to tumor growth by causing inflammation, which may stimulate nearby tumor cells to grow and invade. As a result, senescent tumor cells can cause chronic inflammation and contribute to the development of resistance to chemotherapy and targeted therapies. Moreover, after the completion of chemotherapy or targeted therapy, tumor cells can escape senescence, acquire stem cell characteristics, and exhibit a greater tumor-initiating potential in vivo (21). Therefore, a major knowledge gap for clinical translation will be a critical evaluation of whether the induction and promotion of senescence are specific to certain anti-cancer agents, and whether the occurrence of senescence is uniform or restricted to certain tumors and tumor lineages, particularly tumors of specific oncogenotypes.
Use of well-defined markers of cellular senescence
Examination of tumor cell senescence is challenging because there is no single well-defined marker for senescence. Moreover, The Cancer Genome Atlas (TGCA) data analysis can yield information on single high mRNA expression of CDKN2A (p16INK4a), CDKN1A (p21WAF1/Cip1) and MKI67 (Ki67) but not for lipofuscin, another hallmark of senescence (22). Because lipofuscin results from non-degradable aggregates of oxidized lipids and proteins that accumulate in lysosomes of senescent cells due to senescence-related lysosomal malfunction (22), there is no corresponding coding gene for lipofuscin. Due to these reasons, the data on whether senescence occurs in response to anti-cancer therapy, including chemotherapy and radiotherapy in the clinic are sparse (13). Two previous studies using beta-galactosidase staining as a senescence marker, observed induction of senescence in p53 null human lung cancer cells after continuous exposure to 400 nM 5-azacytidine and after carboplatin plus docetaxel treatment, but not in untreated cells (3,23). The current study, by using well-defined markers of cellular senescence, sheds new light on the presence of senescent cells in NSCLCs and provides a model for future clinical translational studies.
Clinical trials and preclinical models for cellular senescent targeted therapy
One of the major mechanisms by which senescent cells remain viable for a longer period as well as acquire resistance to genotoxic anti-cancer agents is due to their intrinsic ability to upregulate one or more senescent cell anti-apoptotic pathways (SCAPs). Therefore, the timely elimination of senescent cancer cells by using senolytic drugs (drugs targeting senescent cells specifically) or “senomorphics” (drugs targeting the SASP secretome) could potentially benefit cancer control (Figure 1). Senolytic compounds that target either several or single SCAP pathways are in different phases of clinical trials (24). Of significant importance, none of the current trials are directed as cancer therapeutic trials. It has been shown that targeting senescence-like fibroblasts radio sensitizes NSCLC and reduces radiation-induced pulmonary fibrosis (25). However, extensive pre-clinical studies are needed to not only identify specific senolytic compound(s) that targets different tumor types but also to determine the side effects caused by both senolytic compounds and the elimination of senescent cells in healthy organs. In this regard, there is a need to develop preclinical models, particularly with human tumors, to test the efficacy of senolytic and senomorphic therapies in specific cancer lineages, the oncogenotypes, and combined with different, chemotherapy, targeted therapy, immune checkpoint blockade, and radiotherapy approaches.
Acknowledgments
Funding: This study was supported in part by the University of Texas Lung Cancer Specialized Program in Research Excellence (SPORE, P50-CA-070907), and the National Institute of Allergy and Infectious Disease (No. 1U01AI156189-01).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-678/coif). JDM receives licensing fees from the National Institutes of Health and University of Texas Southwestern for distribution of human tumors and normal cell lines. JWS has an ownership interest (including patents) in and has an unpaid consultant/advisory board relationship with Maia Biotechnology. AA has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fumagalli M, d'Adda di Fagagna F. SASPense and DDRama in cancer and ageing. Nat Cell Biol 2009;11:921-3. [Crossref] [PubMed]
- Maciejowski J, Li Y, Bosco N, et al. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell 2015;163:1641-54. [Crossref] [PubMed]
- Ewald JA, Desotelle JA, Wilding G, et al. Therapy-induced senescence in cancer. J Natl Cancer Inst 2010;102:1536-46. [Crossref] [PubMed]
- White RR, Vijg J. Do DNA Double-Strand Breaks Drive Aging? Mol Cell 2016;63:729-38. [Crossref] [PubMed]
- Mukherjee S, Abdisalaam S, Bhattacharya S, et al. Mechanistic link between DNA damage sensing, repairing and signaling factors and immune signaling. Adv Protein Chem Struct Biol 2019;115:297-324. [Crossref] [PubMed]
- Abdisalaam S, Mukherjee S, Bhattacharya S, et al. NBS1-CtIP-mediated DNA end resection suppresses cGAS binding to micronuclei. Nucleic Acids Res 2022;50:2681-99. [Crossref] [PubMed]
- Abdisalaam S, Bhattacharya S, Mukherjee S, et al. Dysfunctional telomeres trigger cellular senescence mediated by cyclic GMP-AMP synthase. J Biol Chem 2020;295:11144-60. [Crossref] [PubMed]
- Takahashi A, Loo TM, Okada R, et al. Downregulation of cytoplasmic DNases is implicated in cytoplasmic DNA accumulation and SASP in senescent cells. Nat Commun 2018;9:1249. [Crossref] [PubMed]
- Sun L, Wu J, Du F, et al. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013;339:786-91. [Crossref] [PubMed]
- Kumari R, Jat P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol 2021;9:645593. [Crossref] [PubMed]
- Chandra T, Ewels PA, Schoenfelder S, et al. Global reorganization of the nuclear landscape in senescent cells. Cell Rep 2015;10:471-83. [Crossref] [PubMed]
- Saleh T, Tyutynuk-Massey L, Cudjoe EK Jr, et al. Non-Cell Autonomous Effects of the Senescence-Associated Secretory Phenotype in Cancer Therapy. Front Oncol 2018;8:164. [Crossref] [PubMed]
- Prasanna PG, Citrin DE, Hildesheim J, et al. Therapy-Induced Senescence: Opportunities to Improve Anticancer Therapy. J Natl Cancer Inst 2021;113:1285-98. [Crossref] [PubMed]
- Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015;14:644-58. [Crossref] [PubMed]
- Prata LGPL, Ovsyannikova IG, Tchkonia T, et al. Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol 2018;40:101275. [Crossref] [PubMed]
- Song P, An J, Zou MH. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020;9:671. [Crossref] [PubMed]
- Kale A, Sharma A, Stolzing A, et al. Role of immune cells in the removal of deleterious senescent cells. Immun Ageing 2020;17:16. [Crossref] [PubMed]
- Domen A, Deben C, De Pauw I, et al. Prognostic implications of cellular senescence in resected non-small cell lung cancer. Transl Lung Cancer Res 2022;11:1526-39. [Crossref] [PubMed]
- Ballman KV. Biomarker: Predictive or Prognostic? J Clin Oncol 2015;33:3968-71. [Crossref] [PubMed]
- Kohli J, Wang B, Brandenburg SM, et al. Algorithmic assessment of cellular senescence in experimental and clinical specimens. Nat Protoc 2021;16:2471-98. [Crossref] [PubMed]
- Roger L, Tomas F, Gire V. Mechanisms and Regulation of Cellular Senescence. Int J Mol Sci 2021;22:13173. [Crossref] [PubMed]
- Gorgoulis V, Adams PD, Alimonti A, et al. Cellular Senescence: Defining a Path Forward. Cell 2019;179:813-27. [Crossref] [PubMed]
- Roberson RS, Kussick SJ, Vallieres E, et al. Escape from therapy-induced accelerated cellular senescence in p53-null lung cancer cells and in human lung cancers. Cancer Res 2005;65:2795-803. [Crossref] [PubMed]
- Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med 2022;28:1556-68. [Crossref] [PubMed]
- Meng J, Li Y, Wan C, et al. Targeting senescence-like fibroblasts radiosensitizes non-small cell lung cancer and reduces radiation-induced pulmonary fibrosis. JCI Insight 2021;6:146334. [Crossref] [PubMed]


